用于大容量储氢的金属修饰亚甲基桥环对苯纳米管
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
高效的储氢对于推进可持续的氢经济至关重要。本研究介绍了一类由亚甲基桥接环对苯乙烯(MCPPs)构成的新型纳米管作为大容量储氢材料。这些纳米管具有五边形环,作为结合三维过渡金属原子的活性位点,避免结构变形或金属聚集。金属修饰诱导可调谐的磁性和电子性能,如铁磁性和带隙减小,增强反应性和吸附行为。在所研究的金属中,钒、镍和铜表现出良好的氢吸附。铜修饰的MCPP纳米管的重量氢容量为8.45 wt%,最佳吸附能为- 0.20 eV,无需低温冷却即可实现室温储存(~ 280 K)。分子动力学模拟证实热稳定性高达500k,确保耐用性和可重用性。这些发现强调了金属修饰的MCPP纳米管作为下一代储氢材料的潜力,它具有高容量、热弹性和实际可重用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
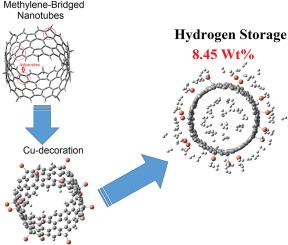
Metal-decorated methylene-bridged cycloparaphenylene nanotubes for high-capacity hydrogen storage
Efficient hydrogen storage is critical for advancing a sustainable hydrogen economy. This study introduces a novel class of nanotubes constructed from methylene-bridged cycloparaphenylenes (MCPPs) as high-capacity hydrogen storage materials. These nanotubes feature pentagonal rings that act as active sites for binding 3d transition metal atoms, avoiding structural deformation or metal clustering. Metal decoration induces tunable magnetic and electronic properties, such as ferromagnetism and band gap reduction, enhancing reactivity and adsorption behavior. Among the studied metals, vanadium, nickel, and copper demonstrate favorable hydrogen adsorption. Copper-decorated MCPP nanotubes achieve a gravimetric hydrogen capacity of 8.45 wt% and an optimal adsorption energy of −0.20 eV, enabling room-temperature storage (∼280 K) without cryogenic cooling. Molecular dynamics simulations confirm thermal stability up to 500 K, ensuring durability and reusability. These findings underscore the potential of metal-decorated MCPP nanotubes as promising candidates for next-generation hydrogen storage, combining high capacity, thermal resilience, and practical reusability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


