高碱煤的净化:合成气生产及氮转化机理研究
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
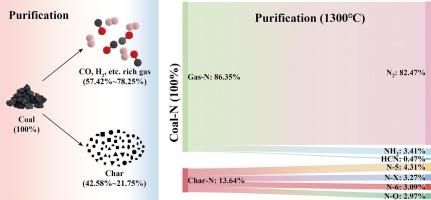
高碱煤是一种广泛存在于许多资源丰富国家的燃料,净化-燃烧策略已成为解决高碱煤清洁和高效利用的双重挑战的有希望的途径。本研究在一定的净化温度(Tp)范围内,研究了落管炉高温净化HAC过程中碳、氢、氮的迁移和转化机制。结果表明,Tp的增加显著提高了水煤气反应合成气(CO和H2)的产量,促进了燃料从非均相转化到均相转化的转变。在1300℃时,冷气效率达到62.96%。净化还改善了颗粒特性,导致颗粒尺寸减小,微孔隙度增加,气固反应性增强。拉曼光谱显示稳定的石墨结构下降,伴随着缺陷碳框架的增加。此外,高温加速了油气裂解和液气转化,显著提高了燃料c的转化率。在强还原条件下,燃料n向气相氮的转化率达到86.35%,NH3和HCN优先反应生成N2而非NOx。焦中的芳香氮结构在1300°C以上变得热稳定,主要以N-Q的形式存在。不同氮种的转化趋势与温度有关,N-5分解为N-6和N-Q,而N-6则因氧化或挥发而逐渐下降。这些发现为净化过程中燃料n的转化行为提供了新的见解,为净化-燃烧系统中Tp的优化提供了理论支持。研究结果与全球在先进能源系统中部署高效、低排放的煤炭利用技术高度相关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Purification of high alkali coal: Insights into syngas production and nitrogen conversion mechanism
The purification–combustion strategy has emerged as a promising pathway for addressing the dual challenge of clean and efficient utilization of high alkali coal (HAC), a fuel widely present in many resource-rich countries. This study investigates the migration and transformation mechanisms of carbon, hydrogen, and nitrogen during the high-temperature purification of HAC using a drop-tube furnace across a range of purification temperatures (Tp). Results indicate that an increase in Tp significantly enhances syngas (CO and H2) production via the water–gas reaction, promoting the transition from heterogeneous to homogeneous fuel conversion. At 1300 °C, the cold gas efficiency reached 62.96%. The purification also refined particle characteristics, leading to reduced particle size, increased microporosity, and enhanced gas–solid reactivity. Raman spectroscopy revealed a decline in stable graphitic structures, accompanied by an increase in defect carbon frameworks. Furthermore, high temperatures accelerated hydrocarbon cracking and liquid–gas transformation, significantly improving fuel-C conversion. The transformation of fuel-N into gas-phase nitrogen peaked at 86.35% under strong reducing conditions, where NH3 and HCN preferentially reacted to form N2 rather than NOx. Aromatic nitrogen structures in char became thermally stable above 1300 °C, predominantly in the form of N-Q. The conversion trends of different nitrogen species were temperature-dependent, with N-5 decomposing into N-6 and N-Q, while N-6 exhibited a progressive decline due to oxidation or volatilization. These findings offer new insights into fuel-N transformation behavior during purification, providing theoretical support for the optimization of Tp in purification–combustion systems. The outcomes are highly relevant to the global deployment of high-efficiency, low-emission coal utilization technologies in advanced energy systems.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


