碳酸盐含盐含水层CO2捕获的反应输运模型:润湿性、温度和盐度对矿化和储存效率的耦合影响
IF 7.5
1区 工程技术
Q2 ENERGY & FUELS
引用次数: 0
摘要
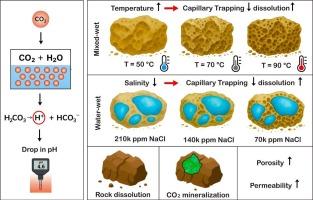
要在含盐含水层中安全地长期储存二氧化碳,就需要清楚地了解储层条件和地球化学反应如何影响岩石性质和捕获机制。虽然已经研究了润湿性、温度和盐度的单独影响,但它们的综合影响,特别是在不同的润湿性状态下,滞后性控制相行为,仍然没有充分量化。本研究采用反应传输模型来评估温度(50-90°C)、盐度(7万- 21万ppm NaCl)和润湿性对富碳酸盐地层中CO2矿化、溶解和毛细管捕获的影响。方解石、高岭石和钙长石的地球化学反应使用过渡状态理论建模,而水湿和混合湿条件则通过相对渗透率和毛细管压力曲线来表示,这些曲线捕捉了与润湿性相关的流动行为。从50°C到90°C,混合湿体系的矿化从~ 4.4 × 106 mol增加到~ 1.3 × 107 mol,而毛细管捕获从~ 67%减少到~ 54%(水湿),从~ 48%减少到~ 36%(混合湿),溶解上升到30%。在90°C时,盐度的增加使CO2羽流从混合湿体系中垂直拉长、以溶解为主(~ 36%)转变为水湿体系中侧向受限、以毛细血管为主(~ 82%),受迟滞(0.2 vs. 0.35)控制。方解石的降水量随着温度的升高而下降~ 22%(水湿),随着盐度的升高而下降~ 14%,而高岭石的降水量增加两倍,钙长石的溶解度根据润湿性在- 25%到+ 90%之间变化。60多年来,孔隙度和渗透率分别适度增加~ 0.36%和~ 1.22%。这些发现阐明了润湿性驱动的界面动力学如何影响矿化和相俘获,为优化不同储层环境下的二氧化碳储存性能提供了机制指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Reactive transport modeling of CO2 trapping in carbonate saline aquifers: Coupled effects of wettability, temperature, and salinity on mineralization and storage efficiency
Secure long-term storage of carbon dioxide (CO2) in saline aquifers requires a clear understanding of how reservoir conditions and geochemical reactions influence rock properties and trapping mechanisms. While the individual effects of wettability, temperature, and salinity have been examined, their combined impact, particularly under varying wettability states where hysteresis controls phase behavior, remains insufficiently quantified. This study employs reactive transport modeling to assess the effects of temperature (50–90 °C), salinity (70,000–210,000 ppm NaCl), and wettability on CO2 mineralization, dissolution, and capillary trapping in carbonate-rich formations. Geochemical reactions involving calcite, kaolinite, and anorthite are modeled using transition state theory, while water-wet and mixed-wet conditions are represented through relative permeability and capillary pressure curves that capture wettability-dependent flow behavior. Mineralization increases from ∼ 4.4 × 106 to ∼ 1.3 × 107 mol in mixed-wet systems from 50 °C to 90 °C, while capillary trapping decreases from ∼ 67 % to ∼ 54 % (water-wet) and ∼ 48 % to ∼ 36 % (mixed-wet), and dissolution rises to > 30 %. At 90 °C, increasing salinity shifts CO2 plumes from vertically elongated, dissolution-dominant (∼36 %) in mixed-wet to laterally confined, capillary-dominant (∼82 %) in water-wet systems, governed by hysteresis (0.2 vs. 0.35). Calcite precipitation declines by ∼ 22 % (water-wet) with temperature and by ∼ 14 % with salinity, while kaolinite precipitation triples and anorthite dissolution varies from − 25 % to + 90 % depending on wettability. Over 60 years, porosity and permeability increase modestly by ∼ 0.36 % and ∼ 1.22 %. These findings clarify how wettability-driven interfacial dynamics influence mineralization and phase trapping, providing mechanistic guidance for optimizing CO2 storage performance across diverse reservoir settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fuel
工程技术-工程:化工
CiteScore
12.80
自引率
20.30%
发文量
3506
审稿时长
64 days
期刊介绍:
The exploration of energy sources remains a critical matter of study. For the past nine decades, fuel has consistently held the forefront in primary research efforts within the field of energy science. This area of investigation encompasses a wide range of subjects, with a particular emphasis on emerging concerns like environmental factors and pollution.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


