酚酸抑制α-淀粉酶的机理研究:结合酶分析、光谱学和分子对接
IF 4
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
2型糖尿病(T2D)是一种以持续高血糖和胰岛素抵抗为特征的慢性代谢紊乱。抑制α-淀粉酶活性是缓解餐后高血糖的关键策略。本研究考察了结构不同的酚酸——苯甲酸型和肉桂酸型对α-淀粉酶的抑制作用。其中对羟基苯甲酸(PHBA)和阿魏酸(FA)表现出较强的抑制活性。PHBA (IC₅₀= 3.08 μg/mL)的抑制作用强于FA (76.73 μg/mL)和阿卡波糖(16.7 μg/mL)。动力学分析显示混合型抑制以竞争结合为主。光谱分析(紫外、荧光、CD、FT-IR)表明PHBA通过氢键更有效地改变酶的构象。分子对接和动力学模拟证实了PHBA具有更强的结合亲和力(FA为- 4.94 kcal/mol > - 4.34 kcal/mol)和α-淀粉酶活性位点更强的相互作用稳定性。这些发现为酚酸的构效关系提供了机制上的见解,并支持了它们在开发血糖控制功能食品中的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
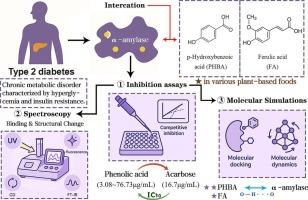
Mechanistic study of phenolic acid inhibition of α-amylase: combining enzyme assays, spectroscopy, and molecular docking
Type 2 diabetes (T2D) is a chronic metabolic disorder marked by persistent hyperglycemia and insulin resistance. Inhibiting α-amylase activity is a key strategy to mitigate postprandial hyperglycemia. This study investigated the inhibitory effects of structurally distinct phenolic acids—benzoic acid-type and cinnamic acid-type on α-amylase. Among them, p-hydroxybenzoic acid (PHBA) and ferulic acid (FA) showed superior inhibitory activity. PHBA (IC₅₀ = 3.08 μg/mL) demonstrated stronger inhibition than FA (76.73 μg/mL) and acarbose (16.7 μg/mL). Kinetic analysis revealed mixed-type inhibition dominated by competitive binding. Spectroscopic analyses (UV, fluorescence, CD, FT-IR) indicated that PHBA more effectively altered enzyme conformation through hydrogen bonding. Molecular docking and dynamics simulations confirmed PHBA's stronger binding affinity (−4.94 kcal/mol > −4.34 kcal/mol for FA) and greater interaction stability at the α-amylase active site. These findings provide mechanistic insight into structure–activity relationships of phenolic acids and support their potential in the development of functional foods for glycemic control.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


