炎症条件下人类结肠膜衍生单层的功能和形态学特征
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
尽管炎症性肠病(IBD)的诊断和治疗取得了进展,但其发病率和患病率仍在上升。人类临床前模型的缺乏限制了研究疾病机制和开发有效疗法的能力。人类结肠体提供了一个生理相关的体外系统,密切模仿结肠上皮。在本研究中,我们利用结肠腺衍生的单层建立了体外IBD模型,并通过暴露于TNF-α和IFN-γ诱导炎症。这种治疗显著损害了上皮屏障的完整性,增加了炎症介质的分泌,如IL-8和CCL20。尽管细胞代谢活性在很大程度上得以保留,但观察到细胞毒性作用:共聚焦显微镜也显示细胞凋亡增加,LGR5 mRNA转录降低表明上皮更新受损。在功能上,炎症导致阿替洛尔的细胞旁转运显著增加,而心得安的转运不受影响,这突出了肠道炎症对口服药物吸收和生物利用度的影响。该模型为研究ibd相关的上皮功能障碍和评估潜在的治疗干预提供了一个强大的平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
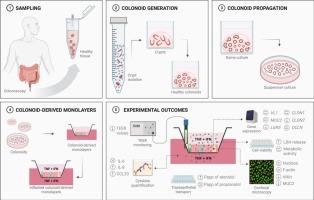
Functional and morphological characterisation of human colonoid-derived monolayers under inflammatory conditions
Despite progress in the diagnosis and treatment of inflammatory bowel disease (IBD), its incidence and prevalence continue to rise. The lack of human preclinical models limits the ability to study disease mechanisms and develop effective therapies. Human colonoids provide a physiologically relevant in vitro system that closely mimics the colonic epithelium. In this study, we established an in vitro IBD model using colonoid-derived monolayers and induced inflammation through exposure to TNF-α and IFN-γ. This treatment significantly impaired epithelial barrier integrity and increased the secretion of inflammatory mediators, like IL-8 and CCL20. Although cell metabolic activity was largely preserved, a cytotoxic effect was observed: the increased apoptosis, revealed also by confocal microscopy, and a compromised epithelial renewal, suggested by lower LGR5 mRNA transcription, were evidenced. Functionally, inflammatory conditions led to a significant increase in the paracellular transport of atenolol, while the transport of propranolol remained unaffected, highlighting the impact of intestinal inflammation on oral drug absorption and bioavailability. This model provides a robust platform for studying IBD-related epithelial dysfunctions and evaluating potential therapeutic interventions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


