含紫杉醇的抗菌近红外靶向纳米复合水凝胶用于骨修复
IF 7.9
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
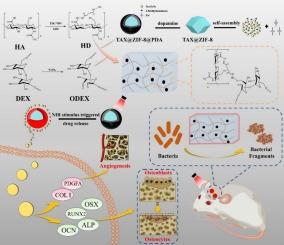
骨缺损一直是创伤骨科的一大难题。尽管骨组织工程取得了进步,但在开发毒性更小、更有效、减少二次手术需求的骨植入物方面仍然存在瓶颈。为了解决这个问题,我们设计了一种光响应骨靶向智能纳米输送系统。该系统将聚多巴胺(PDA)包覆的负载税的ZIF-8纳米颗粒集成到具有强生物粘附性和生物相容性的水凝胶中(TAX@ZIF-8@PDA/ODEX-HD, TZPG)。当暴露在近红外光下时,TZPG + NIR的光热转换效率高达51.79%。它对金黄色葡萄球菌的抑制率为99%,同时还能增强成骨细胞(MC3T3-E1)的增殖和分化,从而加速大鼠颅骨骨缺损模型的骨缺损修复。此外,TZPG + NIR通过上调成骨相关蛋白(ALP、RUNX2、OSX、OCN),同时提高col - 1和PDGFA蛋白的表达,促进血管生成,从而加速骨组织修复。总的来说,TZPG + NIR在体外创造了一个抗菌微环境,在骨修复周期中实现了智能控释和程序化降解,实现了一次性的治疗效果。本研究强调了近红外光响应材料TZPG在再生医学中的应用前景,为开发多功能骨支架修复骨缺损提供了一种新的途径,可作为自体骨移植的可行替代方案。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Antibacterial NIR responsive targeted nanocomposite hydrogels loaded with Taxifolin for bone repair
Bone defects have long posed a challenge in trauma orthopedics. Despite advancements in bone tissue engineering, there remains a bottleneck in developing bone implants that are less toxic, more effective, and reduce the need for secondary surgeries. To address this, we devised a light-responsive bone-targeted smart nano-delivery system. This system integrates polydopamine (PDA)-coated TAX-loaded ZIF-8 nanoparticles into a hydrogel with strong bioadhesion and biocompatibility (TAX@ZIF-8@PDA/ODEX-HD, TZPG). When exposed to near-infrared light, TZPG + NIR demonstrated a high photothermal conversion efficiency of 51.79 %. It exhibited a 99 % inhibition rate against Staphylococcus aureus, while also enhancing the proliferation and differentiation of osteoblasts (MC3T3-E1), thereby accelerating bone defect repair in a rat cranial bone defect model. Additionally, TZPG + NIR accelerates bone tissue repair by upregulating osteogenic-related proteins (ALP, RUNX2, OSX, OCN) while enhancing the expression of COL-I and PDGFA proteins to promote angiogenesis. Overall, TZPG + NIR created an antimicrobial microenvironment in vitro, enabled intelligent controlled release, and programmed degradation during the bone repair cycle, achieving a one-time therapeutic effect. This study highlights the promising applications of near-infrared light-responsive material TZPG in regenerative medicine, offering a novel approach to developing multifunctional bone scaffolds for repairing bone defects as a viable alternative to autografts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Materials & Design
Engineering-Mechanical Engineering
CiteScore
14.30
自引率
7.10%
发文量
1028
审稿时长
85 days
期刊介绍:
Materials and Design is a multi-disciplinary journal that publishes original research reports, review articles, and express communications. The journal focuses on studying the structure and properties of inorganic and organic materials, advancements in synthesis, processing, characterization, and testing, the design of materials and engineering systems, and their applications in technology. It aims to bring together various aspects of materials science, engineering, physics, and chemistry.
The journal explores themes ranging from materials to design and aims to reveal the connections between natural and artificial materials, as well as experiment and modeling. Manuscripts submitted to Materials and Design should contain elements of discovery and surprise, as they often contribute new insights into the architecture and function of matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


