聚酰胺膜表面官能团相关有机污染的分子动力学研究
IF 9.8
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
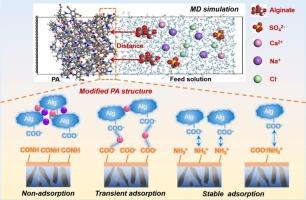
在许多应用中,有机污染对聚酰胺反渗透(RO)膜造成了重大限制。过去的大量工作主要是提供宏观尺度的实验验证;然而,对现有的离子结垢机理的深入了解仍处于起步阶段。本文利用分子动力学(MD)模拟,研究了反渗透膜表面的特定表面官能团(- cooh、- nh2和- conh -)如何起作用并导致有机污染。我们的研究结果表明,官能团引发的有机污染主要归因于静电相互作用,其次是离子键、氢键和疏水相互作用。密度泛函理论(DFT)计算显示,含有羧基的反渗透体系对膜表面的典型污染物(如海藻酸盐)具有“桥接效应”,而氨基与溶液中的海藻酸盐结合的可能性甚至更高,两者都受到静电相互作用的显著影响,加剧了有机污染。相比之下,具有整齐酰胺键的假设反渗透系统被证明具有最少的膜表面与污染物之间的相互作用,从而显著增强了抗污染能力。总的来说,我们的模拟为阐明分子水平的污染机制提供了一个动态框架,这可能会引导未来防污反渗透膜的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Surface functional groups related organic fouling on polyamide membranes: A molecular dynamics study
In many applications, organic fouling poses a significant limitation to polyamide reverse osmosis (RO) membranes. Extensive efforts in the past have mostly provided macroscopic-scale experimental validations; however, a deep understanding of the existing co-ion fouling mechanism is still in its infancy. Herein, using molecular dynamics (MD) simulations, we report how specific surface functional groups (–COOH, –NH2, and –CONH–) on the RO membrane surface work and contribute to organic fouling. Our results indicate that functional group-initiated organic fouling is mainly ascribed to electrostatic interactions, supplemented by ionic bonding, hydrogen bonding, and hydrophobic interactions. Density functional theory (DFT) calculations revealed the RO system containing carboxylic acid groups was subject to a “bridge-effect” to typical foulants (e.g., alginate) on the membrane surface, whereas the amino groups had an even increased probability of bonding to alginate in solution, both of which were markedly influenced by the electrostatic interaction, exacerbating the organic fouling. In contrast, a hypothetical RO system with neat amide linkages was proven to have the least number of interactions between the membrane surface and contaminants, resulting in notably enhanced fouling resistance. Overall, our simulations offer a dynamic framework for elucidating molecular-level fouling mechanisms, which may navigate the future development of antifouling reverse osmosis membranes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Desalination
工程技术-工程:化工
CiteScore
14.60
自引率
20.20%
发文量
619
审稿时长
41 days
期刊介绍:
Desalination is a scholarly journal that focuses on the field of desalination materials, processes, and associated technologies. It encompasses a wide range of disciplines and aims to publish exceptional papers in this area.
The journal invites submissions that explicitly revolve around water desalting and its applications to various sources such as seawater, groundwater, and wastewater. It particularly encourages research on diverse desalination methods including thermal, membrane, sorption, and hybrid processes.
By providing a platform for innovative studies, Desalination aims to advance the understanding and development of desalination technologies, promoting sustainable solutions for water scarcity challenges.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


