不对称aza-BODIPY衍生物近红外双光子吸收响应及激发态动力学评价
IF 4.6
2区 化学
Q1 SPECTROSCOPY
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy
Pub Date : 2025-10-01
DOI:10.1016/j.saa.2025.127010
引用次数: 0
摘要
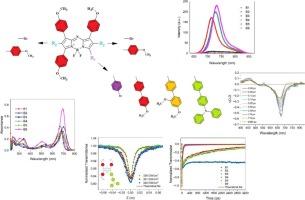
研究了不对称氮杂- bodipy衍生物,分别在氮杂- bodipy骨架的2,6和β位上分别修饰了4-溴苯基(B1)、4-溴和4-溴苯基(B2)、4-N, n -二苯基氨基联苯(B3)、4-甲氧基联苯(B4)、4-甲氧基苯基和4-溴苯基(B5)和2,4-二甲氧基联苯(B6)基团。对化合物进行了深入的研究,以了解它们的线性光学性质、近红外非线性吸收响应和激发态动力学。研究了取代基和溶剂(THF, CHCl3)对化合物的线性吸收和荧光行为的影响。化合物B1和B4对THF有较高的吸收,而化合物B5对CHCl3有较高的吸收。化合物B2、B3和B6未见明显变化。荧光光谱显示,化合物B1、B4和B6在CHCl3介质中的荧光量子产率比在THF中更高。B2、B3和B5的荧光被明显猝灭,这是由于给电子的二苯胺单元和甲氧基以及溴原子的重原子效应对分子内电荷转移(ICT)效应的影响。用泵浦探测光谱分析了化合物的激发态动力学。在1000 nm处观察到所有化合物的近红外双光子吸收(TPA)响应。化合物B3的TPA截面(TPCS)最高,为210 GM,这是由于二苯基氨基联苯单元具有较高的ICT能力。化合物B3和B6具有较强的光物理性质;B3具有较高的ICT活性和最高的TPCS,而B6具有较高的荧光量子产率。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Evaluation of near-infrared two-photon absorption response and excited state dynamics of asymmetric aza-BODIPY derivatives
The study focused on asymmetric aza-BODIPY derivatives, which were modified with 4-bromophenyl (B1), 4-bromo and 4-bromophenyl (B2), 4-N,N-diphenylaminobiphenyl (B3), 4-methoxybiphenyl (B4), 4-methoxyphenyl and 4-bromophenyl (B5), and 2,4-dimethoxybiphenyl (B6) groups at 2,6 and β positions of aza-BODIPY skeleton. The compounds were thoroughly investigated to understand their linear optical properties, near-infrared nonlinear absorption responses, and dynamics of excited states. The linear absorption and fluorescence behaviors of the compounds were studied depending on the substituents and solvents (THF, CHCl3). Compounds B1 and B4 showed higher absorption in THF, while compound B5 exhibited higher absorption in CHCl3. No significant change was observed in compounds B2, B3, and B6. The fluorescence spectra revealed that compounds B1, B4, and B6 exhibit comparatively higher fluorescence quantum yields in CHCl3 medium than in THF. The fluorescence of B2, B3, and B5 was significantly quenched due to the strong intramolecular charge transfer (ICT) effects influenced by the electron-donating diphenylamine unit and methoxy groups, as well as the heavy atom effect of bromine atoms. Excited state dynamics of all compounds were revealed with pump-probe spectroscopy. Near-infrared two-photon absorption (TPA) response at 1000 nm was observed for all the studied compounds. Compound B3 indicated the highest TPA cross-section (TPCS) (210 GM) due to high ICT capability of diphenylaminobiphenyl unit. Compounds B3 and B6 stand out with their strong photophysical properties; B3 exhibited high ICT activity and the highest TPCS, while B6 had high fluorescence quantum yield.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
11.40%
发文量
1364
审稿时长
40 days
期刊介绍:
Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy (SAA) is an interdisciplinary journal which spans from basic to applied aspects of optical spectroscopy in chemistry, medicine, biology, and materials science.
The journal publishes original scientific papers that feature high-quality spectroscopic data and analysis. From the broad range of optical spectroscopies, the emphasis is on electronic, vibrational or rotational spectra of molecules, rather than on spectroscopy based on magnetic moments.
Criteria for publication in SAA are novelty, uniqueness, and outstanding quality. Routine applications of spectroscopic techniques and computational methods are not appropriate.
Topics of particular interest of Spectrochimica Acta Part A include, but are not limited to:
Spectroscopy and dynamics of bioanalytical, biomedical, environmental, and atmospheric sciences,
Novel experimental techniques or instrumentation for molecular spectroscopy,
Novel theoretical and computational methods,
Novel applications in photochemistry and photobiology,
Novel interpretational approaches as well as advances in data analysis based on electronic or vibrational spectroscopy.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


