用于食品安全致癌物质丙烯酰胺和甲醛监测的绿色电化学生物传感器
IF 4.5
2区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
食品安全监测需要检测化学危害的实用策略,如丙烯酰胺(AA)和甲醛(FA),这两种物质都被列为可能的人类致癌物,饮食暴露量往往超过可容忍的限度。传统的方法包括气相色谱-质谱和高效液相色谱,虽然敏感,但成本高,劳动密集,不适合快速现场检测。在这里,我们报道了用于AA和FA检测的绿色电化学生物传感器,该传感器在一次性丝网印刷碳电极(spce)上集成了环保石墨烯和1-芘丁酸n-羟基琥珀酰胺酯(PyrNHS)。在乙醇-水体系中通过液相剥离法制备了石墨烯,制备了具有丰富官能团的少层结构,以稳定的生物受体附着。PyrNHS实现了非共价锚定和定向固定化血红蛋白(Hb)用于AA和甲醛脱氢酶(FDH)用于FA,克服了昂贵和不稳定的纳米材料的局限性。AA生物传感器采用差分脉冲伏安法,在0 ~ 25 μM范围内检测限为4.39 μM。FA生物传感器采用酶促信号机制和计时电流法,在0.1-0.6 mM范围内达到0.02 mM的检测限。两种传感器在速溶咖啡中均具有高选择性、重复性(RSD < 1.3%)和有效性能,与烘焙化学一致,并通过Nash试验验证。这项工作展示了一种可持续的、低成本的、便携式的医疗点(POC)致癌物监测平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
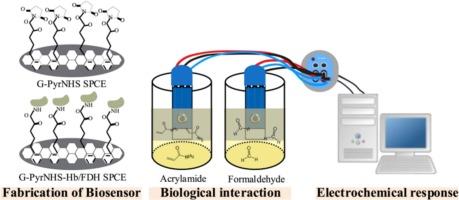
Green electrochemical biosensor for food safety monitoring of carcinogenic acrylamide and formaldehyde
Food safety monitoring demands practical strategies for detecting chemical hazards such as acrylamide (AA) and formaldehyde (FA), both classified as probable human carcinogens with dietary exposures often exceeding tolerable limits. Conventional methods including GC–MS and HPLC, while sensitive, are costly, labour-intensive, and unsuitable for rapid on-site testing. Here, we report green electrochemical biosensors for AA and FA detection that integrate environmentally friendly graphene with 1-pyrenebutyric acid N-hydroxysuccinamide ester (PyrNHS) on disposable screen-printed carbon electrodes (SPCEs). Graphene was synthesized via liquid-phase exfoliation in an ethanol–water system, producing few-layer structures with abundant functional groups for stable bioreceptor attachment. PyrNHS enabled noncovalent anchoring and oriented immobilization of hemoglobin (Hb) for AA and formaldehyde dehydrogenase (FDH) for FA, overcoming limitations of costly and unstable nanomaterials. The AA biosensor operated through a signal-off mechanism using differential pulse voltammetry, with a detection limit of 4.39 μM over 0–25 μM. The FA biosensor employed a signal-on enzymatic mechanism with chronoamperometry, achieving 0.02 mM detection limit across 0.1–0.6 mM. Both sensors showed high selectivity, reproducibility (RSD <1.3 %), and effective performance in instant coffee, consistent with roasting chemistry and validated by Nash assay. This work demonstrates a sustainable, low-cost, and portable platform for point-of-care (POC) carcinogen monitoring.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioelectrochemistry
生物-电化学
CiteScore
9.10
自引率
6.00%
发文量
238
审稿时长
38 days
期刊介绍:
An International Journal Devoted to Electrochemical Aspects of Biology and Biological Aspects of Electrochemistry
Bioelectrochemistry is an international journal devoted to electrochemical principles in biology and biological aspects of electrochemistry. It publishes experimental and theoretical papers dealing with the electrochemical aspects of:
• Electrified interfaces (electric double layers, adsorption, electron transfer, protein electrochemistry, basic principles of biosensors, biosensor interfaces and bio-nanosensor design and construction.
• Electric and magnetic field effects (field-dependent processes, field interactions with molecules, intramolecular field effects, sensory systems for electric and magnetic fields, molecular and cellular mechanisms)
• Bioenergetics and signal transduction (energy conversion, photosynthetic and visual membranes)
• Biomembranes and model membranes (thermodynamics and mechanics, membrane transport, electroporation, fusion and insertion)
• Electrochemical applications in medicine and biotechnology (drug delivery and gene transfer to cells and tissues, iontophoresis, skin electroporation, injury and repair).
• Organization and use of arrays in-vitro and in-vivo, including as part of feedback control.
• Electrochemical interrogation of biofilms as generated by microorganisms and tissue reaction associated with medical implants.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


