不同乳化方法制备的核桃油微胶囊:结构、稳定性及释放机理
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
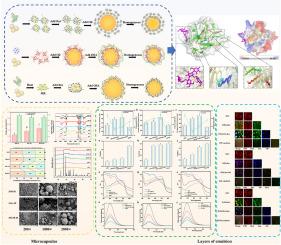
本研究以乳清分离蛋白(WPI)、辛烯基丁二酸酐(OSA)和白藜芦醇(Res)为壁材,分别采用直接混合法(DM)、逐层混合法(LbL)和美拉德复合混合法(MCM)制备核桃油微胶囊。研究了不同乳化方法制备的微胶囊的结构特征、释放特性及分子机理。结果表明,与LbL (LbL- m)和MCM (MCM- m)制备的微胶囊相比,DM (DM- m)制备的微胶囊具有优异的包封性能和氧化稳定性。DM-M包埋率为98.88±0.09%,粒径最小(571.33±24.65 nm),呈光滑均匀的球形结构。在肠消化末期,DM-M的核桃油释放率高达80.92±0.86%,游离脂肪酸释放量为37.90±2.47 μmol/g,说明DM-M的壳在肠消化过程中被完全分解。此外,DM-M的相互作用力大小依次为疏水相互作用(S3-S2: 9.99±0.15 mg/mL)、氢键相互作用(S2-S1: 9.89±0.09 mg/mL)、离子键相互作用(S1: 8.49±0.19 mg/mL)、二硫键相互作用(S4-S3: 2.60±0.07 mg/mL)。LbL-M和MCM-M的疏水相互作用、氢键和离子键的强度除二硫键外均弱于DM-M。三种微胶囊中DM-M的非共价力最强,能形成致密结构,有效防止核桃油液滴聚集。本研究为开发高品质核桃油微胶囊、延长核桃油保质期、包埋脂溶性活性物质提供理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Walnut oil microcapsules prepared with different emulsification methods: Structure, stability, and release mechanism
In the present study, whey protein isolate (WPI), octenyl succinic anhydride (OSA), and resveratrol (Res) were used as wall materials for the preparation of walnut oil microcapsules via the direct mixing method (DM), the layer-by-layer method (LbL), and the Maillard combined mixing method (MCM), respectively. The structural characteristics, release characteristics, and molecular mechanism of microcapsules prepared by different emulsification methods were studied. The results showed that microcapsules prepared by DM (DM-M) exhibit excellent encapsulation performance and oxidation stability compared with microcapsules prepared by LbL (LbL-M) and MCM (MCM-M). The embedding rate of DM-M was 98.88 ± 0.09 %, and the particle size was the smallest (571.33 ± 24.65 nm), showing a smooth and uniform spherical structure. At the end of the intestinal digestion stage, the walnut oil release ratio of DM-M was as high as 80.92 ± 0.86 %, and the free fatty acid release amount was 37.90 ± 2.47 μmol/g, indicating that the shell of DM-M was completely broken down during intestinal digestion. Besides, the order of the interaction forces of DM-M was hydrophobic interaction (S3-S2: 9.99 ± 0.15 mg/mL) > hydrogen bond (S2-S1: 9.89 ± 0.09 mg/mL) > ionic bond (S1: 8.49 ± 0.19 mg/mL) > disulfide bond (S4-S3: 2.60 ± 0.07 mg/mL). The strength of the hydrophobic interaction, hydrogen bond, and ionic bond of LbL-M and MCM-M is weaker than that of DM-M, except for the disulfide bond. DM-M exhibited the strongest non-covalent force of the three microcapsules, which could form a dense structure and effectively prevent walnut oil droplet aggregation. This study provides a theoretical basis for developing high-quality walnut oil microcapsules, extending the shelf life of walnut oil, and embedding fat-soluble active substances.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


