结合蛋白纤维化与动态非共价交联改善高内相乳剂的力学性能及其在空心模型打印中的应用
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究假设高内相乳剂(HIPEs)的3D打印性能将通过蛋白质纤维化和动态非共价交联协同增强。大豆β-甘氨酸(7S)经纤颤修饰后,与三种不同的交联剂(单宁酸(TA)、海藻酸钠(SA)和氯化钙(CaCl2)通过非共价键结合,形成用于3D打印的HIPEs。分子相互作用的测定证实蛋白原纤维(7F)可以分别通过氢键、静电作用和配位键与TA、SA和Ca2+结合。透射电镜显示,蛋白在纤颤后由球形变为枝状树枝状结构,三种交联剂进一步诱导7F聚集,导致二级结构、粒径和表面疏水性发生变化。界面流变学分析表明,SA和TA可以显著降低界面张力,降低7F的渗透和重排速率,有利于形成致密厚的界面膜。Ca2+诱导蛋白质疏水性增加,影响其界面性质,其作用与其他两种交联剂相反。但能有效提高HIPEs的冻融稳定性。与7S相比,由7F形成的HIPEs由于淀粉样纤维缠结而具有明显更高的G′。与交联剂的结合进一步提高了HIPEs的G′。7F-SA形成的HIPEs具有最高的G′、粘度、硬度和相对较低的蠕变值,使得HIPEs在挤压后能够快速恢复结构,从而在空心模型打印中表现出优异的自支撑性能和打印精度。本文章由计算机程序翻译,如有差异,请以英文原文为准。
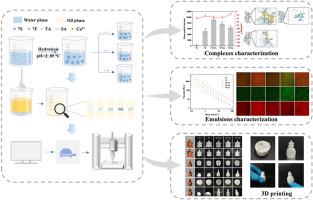
Combining protein fibrosis with dynamic non-covalent crosslink to improve the mechanical properties of high internal phase emulsion and its application in hollow model printing
This study hypothesized that the 3D printing performance of high internal phase emulsions (HIPEs) would be synergistically enhanced by protein fibrosis and dynamic non-covalent crosslink. Soy β-conglycinin (7S) was modified by fibrillation, then combined with three different crosslinking agents (tannic acid (TA), sodium alginate (SA), and calcium chloride (CaCl2)) through non-covalent bonds to form HIPEs for 3D printing. The determination of molecular interaction confirmed that protein fibril (7F) could bond with TA, SA and Ca2+ through hydrogen bond, electrostatic interaction and coordinate bond, respectively. TEM revealed that the shape of protein changed from spherical to branched dendritic structure after fibrillation, three crosslinking agents further induced aggregation of 7F, leading to changes in secondary structure, particle size and surface hydrophobicity. The interfacial rheology analysis suggested that SA and TA could significantly decrease the interfacial tension, the permeation and rearrangement rate of 7F, facilitating the formation of dense and thick interfacial film, as observed in microscopy images. Ca2+ induced an increase in protein hydrophobicity and affected its interfacial properties with opposite effects to the other two crosslinking agents. However, it could effectively improve the freeze-thaw stability of HIPEs. Compared with 7S, HIPEs formed of 7F had substantially higher G′ due to amyloid fibril entanglement. Bonding with crosslinking agents further increased the G′ of HIPEs. HIPEs formed of 7F-SA showed the highest G′, viscosity, hardness and relatively low creep values, which enabled the HIPEs to rapidly recover the structure after extrusion, thus exhibiting excellent self-supporting performance and printing accuracy in hollow model printing.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


