Fe3O4@CQDs/介质阻挡放电等离子体耦合系统降解恩诺沙星:效率、影响因素及协同作用机制
IF 7.1
Q1 ENGINEERING, CHEMICAL
引用次数: 0
摘要
恩诺沙星(ENR)在水生环境中的残留不断增加,加剧了抗生素耐药性,需要有效的降解技术。本文构建了一个Fe3O4@CQDs/介质阻挡放电(DBD)等离子体系统来降解ENR,重点揭示了协同机制。Fe3O4@CQDs是通过水热法合成的,具有增强的比表面积,丰富的表面官能团(羟基,羰基)和高效的Fe(III)/Fe(II)氧化还原循环性能。当放电电压为20 kV, Fe3O4@CQDs投加量为0.3 g/L时,ENR的最佳降解效果为:30 min去除率为95%,协同系数为2.66。该体系对pH波动、Cl⁻、CO32−和腐殖酸具有很强的抗干扰能力。降解中间体和密度泛函理论分析揭示了毒性降低的中间体的降解途径(去氟化、脱羧化、去甲基化)。•OH,•O2−,1O2, ONOO毒血症,H2O2和O3被确认为以e毒血症为引发剂的关键反应物质。Fe3O4@CQDs/DBD的主要协同机制涉及等离子体提供初始能量和活性物质,而Fe3O4@CQDs提供活性位点。此外,Fe(III)/Fe(II)可以驱动Fenton反应生成H2O2,并将O3转化为•OH,抑制e⁻-h⁺的复合,增强氧化作用。此外,Fe3O4@CQDs在4个循环后仍保持77%的活性。这项研究强调了Fe3O4@CQDs/DBD的协同作用,为复杂基质中的抗生素降解提供了可持续的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
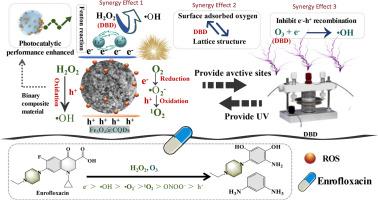
Fe3O4@CQDs/dielectric barrier discharge plasma coupled system for enrofloxacin degradation: efficiency, influencing factors, and synergistic mechanism
The escalating residue of enrofloxacin (ENR) in aquatic environments exacerbates antibiotic resistance, necessitating efficient degradation technologies. Herein, a Fe3O4@CQDs/dielectric barrier discharge (DBD) plasma system was constructed to degrade ENR, with a focus on unraveling the synergistic mechanism. Fe3O4@CQDs are synthesized via the hydrothermal method and exhibit enhanced specific surface area, abundant surface functional groups (hydroxyl, carbonyl), and efficient Fe(III)/Fe(II) redox cycling performance. Optimal degradation of ENR was achieved at 20 kV discharge voltage, 0.3 g/L Fe3O4@CQDs dosage, with a 95% removal efficiency at 30 min and a synergistic factor of 2.66. The system showed robust anti-interference against pH fluctuation, Cl⁻, , and humic acid. Degradation intermediates and density functional theory analysis revealed degradation pathways (defluorination, decarboxylation, demethylation) with intermediates of reduced toxicity. •OH, •, 1O2, ONOO⁻, H2O2, and O3 were confirmed key reactive species with e⁻ as the initiator. The primary synergistic mechanisms of Fe3O4@CQDs/DBD involve the plasma providing initial energy and active species, while Fe3O4@CQDs offers active sites. Additionally, Fe(III)/Fe(II) can drive the Fenton reaction for H2O2 generation and the conversion of O3 to •OH, inhibiting e⁻-h⁺ recombination and enhancing oxidation. Besides, Fe3O4@CQDs retained 77% activity after 4 cycles. This study highlights the Fe3O4@CQDs/DBD synergism, offering a sustainable strategy for antibiotic degradation in complex matrices.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Journal Advances
Engineering-Industrial and Manufacturing Engineering
CiteScore
8.30
自引率
0.00%
发文量
213
审稿时长
26 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


