反常Wickerhamomyces anomalus是Castanea sp . ink致病卵菌Phytophthora cinnamomi和P. xcambivora的捕食者。基于形态学证据,给出了一个说明特定作用方式的模型
IF 3
3区 生物学
Q2 MYCOLOGY
引用次数: 0
摘要
栗树疫霉(Phytophthora cinnamomi)和xcambivora是严重的植物病原体,可引起栗树的根腐病,严重威胁栗树这一历史作物。卵菌产生菌丝体,但难以用传统的杀菌剂或bca控制。最近的研究表明,在实验室环境中,一种酵母内生菌Wickerhamomyces anomalus对关键的收获前和收获后真菌病原体具有很强的拮抗作用。这项研究表明,它还能抑制疫霉菌的生长。显微镜观察显示,异常W. anomalus细胞粘附在疫霉菌菌丝上,并在疫霉菌菌丝内发现,在塌陷的区域内积累,可能以菌丝内容物为食。这些相互作用在没有挥发性化合物、铁载体或水解酶的干预下发生,使菌丝壁保持完整。肉桂菌菌丝与反常W.共培养时的SEM和TEM结果显示,在酵母胁迫下,菌丝内形成了大量的菌丝结构。否则,酵母细胞极化,细胞核融合,孢子数量不规则,细胞间桥,表明生殖周期中断。这与P. cinnamomi和W. anomalus识别彼此的信息素,引发类似交配的反应相一致,导致酵母的附着和内化,而不破坏菌丝细胞壁。这种异常W. anomalus特异的作用模式,与以前报道的不同,表明了作为疫霉收获前管理的BCA的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
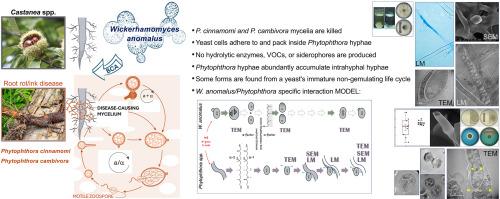
Wickerhamomyces anomalus is a predator of the Castanea spp. ink disease-causing oomycetes Phytophthora cinnamomi and P. xcambivora. Based on morphological evidence, a model illustrating a specific mode of action is provided
Phytophthora cinnamomi and P. xcambivora are serious phytopathogens, namely causing root rot/ink in chestnut trees, which severely threatens this historical crop. Oomycetes produce mycelium but are hard to control with traditional fungicides or BCAs. Recent research revealed Wickerhamomyces anomalus, a yeast endophyte, as a strong antagonist of key pre- and post-harvest fungal pathogens in laboratory settings. This study shows it also inhibits Phytophthora growth. Microscopy revealed that W. anomalus cells adhere to and are found inside Phytophthora hyphae, accumulating within collapsed areas, possibly nourishing on hyphal contents. These interactions occur without the intervention of volatile compounds, siderophores, or hydrolytic enzymes, leaving hyphal walls intact. SEM and TEM of hyphae from P. cinnamomi when co-cultured with W. anomalus showed numerous intrahyphal structures formed in response to the yeast-imposed stress. Otherwise, the yeast shows polarised cells, nuclei fusion, irregularly numbered spores and intercellular bridges, indicating a disrupted reproductive cycle. This is consistent with P. cinnamomi and W. anomalus recognising each other's pheromones, triggering a mating-like response leading to the yeast's attachment and internalisation without damaging the hyphal cell wall. This W. anomalus specific mode-of-action, different from the ones previously reported, suggests potential as a BCA for the pre-harvest management of Phytophthora.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fungal biology
MYCOLOGY-
CiteScore
5.80
自引率
4.00%
发文量
80
审稿时长
49 days
期刊介绍:
Fungal Biology publishes original contributions in all fields of basic and applied research involving fungi and fungus-like organisms (including oomycetes and slime moulds). Areas of investigation include biodeterioration, biotechnology, cell and developmental biology, ecology, evolution, genetics, geomycology, medical mycology, mutualistic interactions (including lichens and mycorrhizas), physiology, plant pathology, secondary metabolites, and taxonomy and systematics. Submissions on experimental methods are also welcomed. Priority is given to contributions likely to be of interest to a wide international audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


