虹鳟鱼副产品蛋白水解物减轻酒精诱导的肝脏氧化应激和失调的自噬
IF 2.2
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
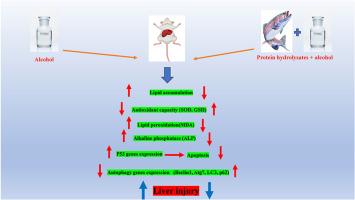
背景:长期饮酒会导致不可逆的肝损伤。虹鳟鱼是天然抗氧化剂的宝贵来源,每年2900万吨的产量中有23%的浪费。本研究探讨了虹鳟鱼蛋白水解物在酒精性脂肪性肝病(AFLD)大鼠模型中的肝脏保护作用,重点关注自噬、凋亡和氧化应激途径。方法雄性大鼠24只,随机分为4组:对照组(C)、酒精组(A)、水解蛋白组(P)、酒精+水解蛋白组(AP)。实验结束时,采集肝组织样本进行分析。评估氧化应激标志物,包括丙二醛(MDA)、超氧化物歧化酶(SOD)和谷胱甘肽(GSH)。此外,我们还评估了肝脏中凋亡相关基因(p53)和自噬相关基因(Beclin1, Atg7, P62)的表达。为了验证基因表达结果,我们还检测了LC3和p53的蛋白表达水平。结果酒精暴露可提高MDA水平,降低SOD活性和GSH。水解液处理通过提高SOD和GSH,降低MDA来恢复抗氧化能力。A组肝脏组织学表现为脂肪变性和糖原降低,P组和AP组肝脏组织学表现明显改善(P小于0.05)。此外,水解物抑制酒精诱导的P53上调和自噬相关基因的调节(p小于0.05)。免疫组织化学显示,AP组P53降低,LC3升高,表明细胞从凋亡向自噬转变,以维持细胞稳态。结论这些结果表明,虹鳟蛋白水解物可能具有治疗酒精性肝损伤的饮食干预潜力,有待进一步验证。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mitigating alcohol-induced liver oxidative stress and dysregulated autophagy with protein hydrolysates derived from rainbow trout by-products
Background
Chronic alcohol consumption causes irreversible liver damage. With 23 % waste from 29 million tons of annual production, rainbow trout is a valuable source of natural antioxidants. This study explores the hepatoprotective effects of rainbow trout protein hydrolysates in an alcohol-induced fatty liver disease (AFLD) rat model, focusing on autophagy, apoptosis, and oxidative stress pathways.
Methods
Twenty-four male rats were divided into four groups: Control (C), alcohol (A), protein hydrolysates (P), and alcohol + protein hydrolysates (AP). At the end of the experiment, liver tissue samples were collected for analysis. Oxidative stress markers, including malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione (GSH), were assessed. Additionally, the expression of genes related to apoptosis (p53) and autophagy (Beclin1, Atg7, P62) in the liver was evaluated. To validate the gene expression results, the protein expression levels of LC3 and p53 were also measured.
Results
Alcohol exposure elevated MDA levels while reducing SOD activity and GSH. Hydrolysate treatment restored antioxidant capacity by enhancing SOD and GSH and lowering MDA. Histology showed hepatic steatosis and reduced glycogen in group A, while groups P and AP exhibited significant improvement (p˂0.05). Additionally, hydrolysates inhibited alcohol-induced P53 upregulation and modulated autophagy-related genes (p˂0.05). Immunohistochemistry showed reduced P53 and increased LC3 in the AP group, indicating a shift from apoptosis to autophagy for cellular homeostasis.
Conclusion
These results suggest that protein hydrolysates derived from rainbow trout may have therapeutic potential as a dietary intervention for managing alcohol-induced liver injury, pending further validation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biochemistry and Biophysics Reports
Biochemistry, Genetics and Molecular Biology-Biophysics
CiteScore
4.60
自引率
0.00%
发文量
191
审稿时长
59 days
期刊介绍:
Open access, online only, peer-reviewed international journal in the Life Sciences, established in 2014 Biochemistry and Biophysics Reports (BB Reports) publishes original research in all aspects of Biochemistry, Biophysics and related areas like Molecular and Cell Biology. BB Reports welcomes solid though more preliminary, descriptive and small scale results if they have the potential to stimulate and/or contribute to future research, leading to new insights or hypothesis. Primary criteria for acceptance is that the work is original, scientifically and technically sound and provides valuable knowledge to life sciences research. We strongly believe all results deserve to be published and documented for the advancement of science. BB Reports specifically appreciates receiving reports on: Negative results, Replication studies, Reanalysis of previous datasets.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


