铝水解制氢:联合实验和第一性原理密度泛函理论研究
IF 11
1区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
摘要
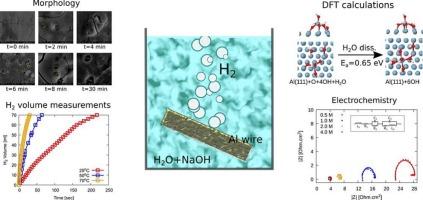
高效、环保的氢气生产是现代能源科学面临的最紧迫的挑战之一。金属上的水水解是一种经济实惠且可持续的制氢方法。铝具有丰度高、成本低、毒性低等优点,是一种很有前途的水解离材料。在这项工作中,我们研究了阻抗测量、XRD、SEM和氢体积测量等方法,研究了铝丝和铝粉在NaOH溶液中的析氢反应。利用第一性原理密度泛函理论(DFT)对实验结果进行了解释。我们的研究结果揭示了反应机理的几个重要方面,如天然氧化物的去除和表面形貌的演变。我们计算设计了一个六步反应机制,描述了表面的侵蚀和Al(OH)4−的释放。我们的研究结果表明,反应在初始阶段以低激活障碍进行,但随着更多的H2O分子在表面吸附和离解,这些障碍增加。我们观察到控制速率决定步骤的因素和计算的激活势垒与实验推导的值很好地吻合。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Hydrolysis of Al for hydrogen production: A joint experimental and first-principles density functional theory investigation
Efficient and environmentally friendly H2 production is one of the most pressing challenges that face modern energy science. Hydrolysis of water over metals is used as an affordable and sustainable method for H2 production. Due to its abundance, low cost, and low toxicity, aluminum (Al) is a promising candidate material to be used for water dissociation. In this work, we investigate using impedance measurements, XRD, SEM, and hydrogen volume measurement methods, the H2 evolution reactions from Al wires and Al powder in a NaOH solution. The experimental results are interpreted with the help of first-principles density functional theory (DFT) calculations. Our results shed light on several important aspects of the reaction's mechanism, such as the removal of the native oxide and the evolution of the surface morphology. We computationally design a six-step reaction mechanism that describes the erosion of the surface and release of Al(OH)4 −. Our results indicate that the reaction proceeds with low activation barriers at the initial stages, but these barriers increase as more H2O molecules adsorb and dissociate on the surface. We observe that factors controlling the rate-determining step, and the computed activation barrier, compare well with the experimentally derived values.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Applied Energy
工程技术-工程:化工
CiteScore
21.20
自引率
10.70%
发文量
1830
审稿时长
41 days
期刊介绍:
Applied Energy serves as a platform for sharing innovations, research, development, and demonstrations in energy conversion, conservation, and sustainable energy systems. The journal covers topics such as optimal energy resource use, environmental pollutant mitigation, and energy process analysis. It welcomes original papers, review articles, technical notes, and letters to the editor. Authors are encouraged to submit manuscripts that bridge the gap between research, development, and implementation. The journal addresses a wide spectrum of topics, including fossil and renewable energy technologies, energy economics, and environmental impacts. Applied Energy also explores modeling and forecasting, conservation strategies, and the social and economic implications of energy policies, including climate change mitigation. It is complemented by the open-access journal Advances in Applied Energy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


