聚多巴胺作为生物相容性和精确的线粒体靶向治疗平台逆转心肌缺血-再灌注损伤
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
靶向线粒体为治疗广泛的主要疾病提供了一种令人信服的策略。然而,开发特异性和生物相容性的线粒体递送载体仍然是一个关键障碍。在这项研究中,我们发现聚多巴胺(PDA)是一种具有内在活性氧(ROS)清除能力的高度生物相容性材料,是一种天然的线粒体靶向生物材料。PDA对几个关键的线粒体外膜蛋白具有很强的结合亲和力,包括电压依赖性阴离子通道和外膜的转座,赋予其固有的线粒体趋向性。作为概念验证,我们构建了一种特殊通道PDA纳米胶囊(CP)来包裹线粒体通透性过渡孔(mPTP)抑制剂环孢素a (CsA),形成CPC。在心肌缺血再灌注损伤(MIRI)模型中,静脉注射CPC选择性地在梗死心肌中积累,并在心肌细胞线粒体内高度富集。CPC不仅抑制了线粒体ROS的爆发,还通过其专门的通道控制释放CsA,抑制mPTP的开放。这种干预通过阻断cGAS-STING途径阻止心肌细胞凋亡并减轻随后的炎症级联。值得注意的是,CPC几乎逆转了MIRI的病理影响,其疗效超过单独使用CsA。这种创新的线粒体靶向方法为线粒体修复提供了一个通用的平台,并为一系列与线粒体损伤相关的疾病提供了新的治疗途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
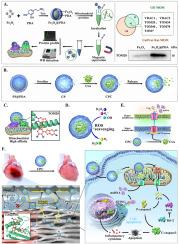
Polydopamine as a biocompatible and precise mitochondrial targeted therapeutic platform for reversing myocardial ischemia-reperfusion injury
Targeting mitochondria offers a compelling strategy for treating a broad spectrum of major diseases. However, the development of specific and biocompatible mitochondrial delivery vectors remains a key obstacle. In this study, we identified polydopamine (PDA)—a highly biocompatible material with inherent reactive oxygen species (ROS)-scavenging capabilities—as a naturally mitochondria-targeting biomaterial. PDA exhibits strong binding affinity to several critical outer mitochondrial membrane proteins, including the voltage-dependent anion channel and translocases of the outer membrane, conferring it with intrinsic mitochondrial tropism. As a proof-of-concept, we constructed a special channel PDA nanocapsule (CP) to encapsulate the mitochondrial permeability transition pore (mPTP) inhibitor cyclosporine A (CsA), forming CPC. In a myocardial ischemia-reperfusion injury (MIRI) model, intravenously administered CPC selectively accumulated in infarcted myocardium and was highly enriched within cardiomyocyte mitochondria. CPC not only suppressed the mitochondrial ROS burst but also released CsA in a controlled manner via its specialized channels, inhibiting mPTP opening. This intervention prevented cardiomyocyte apoptosis and attenuated the subsequent inflammatory cascade by blocking the cGAS-STING pathway. Remarkably, CPC nearly reversed the pathological effects of MIRI, with efficacy surpassing that of CsA alone. This innovative mitochondrial-targeting approach offers a versatile platform for mitochondrial repair and presents new therapeutic avenues for a range of diseases associated with mitochondrial injury.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


