水热法制备新型Dy2MoO6/rGO纳米材料作为增强OER应用的潜在电催化剂
IF 5.4
3区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
为了解决化石燃料过度使用造成的能源问题,人们正在过度研究水电催化技术。水电解通过析氧反应和析氢反应生成氧和氢,但需要良好的催化剂。OER电催化剂由于反应动力学缓慢而限制了水的氧化反应,目前正在努力开发OER电催化剂。在我们的研究中,通过简单的水热法合成了一种新的电催化剂Dy2MoO6/rGO复合材料,以提高OER效率。不同的物理测试,如SEM(扫描电子显微镜),XRD (X射线衍射),FTIR(傅里叶变换红外光谱)和拉曼证实了材料的成功制造。Dy2MoO6/rGO显示出与还原氧化石墨烯(rGO)薄片的通用性,从而增加了纳米催化剂的活性位点和表面可用性。与原始材料相比,Dy2MoO6/rGO复合材料的OER活性有所提高,其过电位(kv)和Tafel相关系数分别为233 mV、35 mV/dec,而Dy2MoO6和rGO分别为287 mV、50 mV/dec和321 mV、70 mV/dec。制备的Dy2MoO6/rGO催化剂在30小时内保持稳定,电流密度下降很小。Dy2MoO6/rGO优异的物理和电化学特性使其在未来成为一种很有前途的电催化剂,用于电气和其他相关应用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
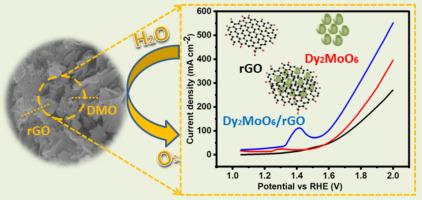
Hydrothermal production of novel Dy2MoO6/rGO nanomaterial as potential electrocatalyst for enhanced OER application
Water electrocatalysis is excessively being investigated for energy production to address the energy issues resulting by excessive use of fossil fuels. Water electrolysis results production of oxygen and hydrogen by oxygen evolution reaction (OER) and hydrogen evolution reaction but it needs a good catalyst. The efforts are being made to develop electrocatalysts for OER which is limiting reaction of water oxidation owing to its sluggish reaction kinetics. In our study, a novel electrocatalyst Dy2MoO6/rGO composite was synthesized via simple hydrothermal method for OER efficiency. Different physical tests like SEM (scanning electron microscope), XRD (X ray diffraction), FTIR (Fourier transform infrared spectroscopy) and Raman confirmed the successful fabrication of materials. The Dy2MoO6/rGO showed versatile with reduced graphene oxide (rGO) sheets resulting in increased active sites and surface availability of nanocatalyst. The Dy2MoO6/rGO composite showed improved OER activity than pristine materials having overpotential (ղ) and Tafel related 233 mV, 35 mV/dec while Dy2MoO6 has (287 mV, 50 mV/dec) and rGO has (321 mV, 70 mV/dec). The fabricated Dy2MoO6/rGO catalyst holds the stability over 30 h with minute decrease in current density. The exceptional physical and electrochemical features of Dy2MoO6/rGO make it a promising electrocatalyst for electrical and other related applications in future.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Inorganic Chemistry Communications
化学-无机化学与核化学
CiteScore
5.50
自引率
7.90%
发文量
1013
审稿时长
53 days
期刊介绍:
Launched in January 1998, Inorganic Chemistry Communications is an international journal dedicated to the rapid publication of short communications in the major areas of inorganic, organometallic and supramolecular chemistry. Topics include synthetic and reaction chemistry, kinetics and mechanisms of reactions, bioinorganic chemistry, photochemistry and the use of metal and organometallic compounds in stoichiometric and catalytic synthesis or organic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


