脱乙酰调控壳聚糖与Pb2+从纳米到宏观的结合机制
IF 11
1区 农林科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
壳聚糖与重金属离子的比结合能力在食品安全中起着至关重要的作用,它与壳聚糖的去乙酰化程度密切相关。然而,控制DD影响的潜在机制仍然不清楚。本文采用实验与模拟相结合的方法研究了壳聚糖与Pb2+在不同DD下的多尺度相互作用。在分子水平上,Pb2+可以通过多个相互作用位点同时螯合壳聚糖的单个链,这种螯合作用随着DD的升高而增强,导致分子的排列更加有序。这种分子排列随后发展为纳米级聚集过程,聚集尺寸、速率和机械稳定性均与DD呈正相关。这种聚集最终导致宏观结构增强,应变和拉应力显著增加2 - 3倍,并随着DD的增加而进一步增加。这些观察到的效应有一个统一的机制:与乙酰基相比,去乙酰化引入了更多的氨基作为更强的螯合位点,从而增强了Pb2+在长度尺度上的结合。这些研究结果为壳聚糖在食品保鲜、抗重金属包装、食品净化等方面的开发和应用提供了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
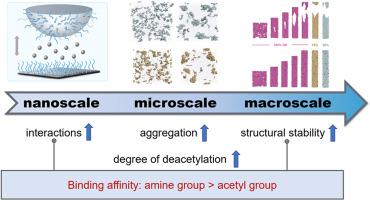
Deacetylation-regulated binding mechanism between chitosan and Pb2+ from nano to macroscale
The specific binding capacity between chitosan and heavy metal ions is critical in food safety, which is closely regulated by its degree of deacetylation (DD). However, the underlying mechanism governing the impact of DD remains unclear. Here we employ a combination of experiments and simulations to study the interaction between chitosan and Pb2+ across multiscale at different DD. At the molecular level, Pb2+ can chelate individual chitosan chains simultaneously via multiple interaction sites, which is enhanced with rising DD and leads to more ordered molecular packing. This molecular arrangement then develops to a nanoscale aggregation process, where the aggregation size, rate, and mechanical stability all show a positive correlation with DD. Such aggregation ultimately leads to enhanced macroscale structures, where the strain and tensile stress are significantly increased by 2 to 3-folds and further rises with increasing DD. These observed effects are governed by a unified mechanism: deacetylation introduces more amino groups that serve as stronger chelation sites than acetyl groups, thereby enhancing Pb2+ binding across length scales. These findings provide foundation for the development and application of chitosan in the food industry, such as food preservation, anti-heavy-metal packaging, and food purification.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food Hydrocolloids
工程技术-食品科技
CiteScore
19.90
自引率
14.00%
发文量
871
审稿时长
37 days
期刊介绍:
Food Hydrocolloids publishes original and innovative research focused on the characterization, functional properties, and applications of hydrocolloid materials used in food products. These hydrocolloids, defined as polysaccharides and proteins of commercial importance, are added to control aspects such as texture, stability, rheology, and sensory properties. The research's primary emphasis should be on the hydrocolloids themselves, with thorough descriptions of their source, nature, and physicochemical characteristics. Manuscripts are expected to clearly outline specific aims and objectives, include a fundamental discussion of research findings at the molecular level, and address the significance of the results. Studies on hydrocolloids in complex formulations should concentrate on their overall properties and mechanisms of action, while simple formulation development studies may not be considered for publication.
The main areas of interest are:
-Chemical and physicochemical characterisation
Thermal properties including glass transitions and conformational changes-
Rheological properties including viscosity, viscoelastic properties and gelation behaviour-
The influence on organoleptic properties-
Interfacial properties including stabilisation of dispersions, emulsions and foams-
Film forming properties with application to edible films and active packaging-
Encapsulation and controlled release of active compounds-
The influence on health including their role as dietary fibre-
Manipulation of hydrocolloid structure and functionality through chemical, biochemical and physical processes-
New hydrocolloids and hydrocolloid sources of commercial potential.
The Journal also publishes Review articles that provide an overview of the latest developments in topics of specific interest to researchers in this field of activity.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


