通过有效的Rh(II)催化的X-H插入反应,扩展了戊二胺基小脑配体的化学空间
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
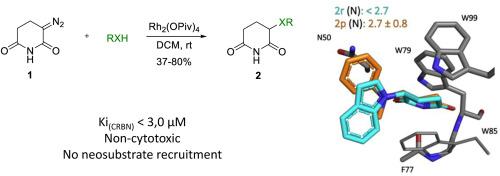
在这项工作中,我们提出了一个简单的一步合成方案,以探索基于谷氨酰胺的小脑(CRBN)配体的靶向蛋白质降解的大化学空间。这是基于我们最近提出的方法,通过Rh(II)催化的3-重氮哌啶-2,6-二酮的X-H插入反应,以中高产率生成结构多样的α -取代戊二胺衍生物系列。共合成了25个具有可变侧链的戊二酰亚胺衍生物并进行了体外评价。所有配体都表现出良好的亲脂性,其中一些配体的结合亲和力可以超过沙利度胺作为参考。此外,大多数化合物在骨髓瘤细胞系和人外周血单核细胞中表现出较低的内在细胞毒性,并且不招募典型的新底物。细胞热移实验进一步证明,最有效的类似物在活细胞中稳定CRBN,证实了它们的靶向作用。该系列的开发伴随着晶体学研究,该研究合理化了观察到的结合亲和力和新底物选择性的改善,并可以支持分子胶活性和PROTACs设计的进一步发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
EXTENDING THE CHEMICAL SPACE OF GLUTARIMIDE-BASED CEREBLON LIGANDS THROUGH AN EFFICIENT Rh(II)-CATALYZED X-H INSERTION REACTION
In this work we present an easy, one-step synthetic protocol to explore a large chemical space of glutarimide-based cereblon (CRBN) ligands for targeted protein degradation. It is built upon our recently suggested approach to generating structurally diverse series of alpha-substituted glutarimide derivatives through an efficient Rh(II)-catalyzed X-H insertion reaction of 3-diazopiperidine-2,6-dione, with moderate to high yields. In total, 25 glutarimide derivatives incorporating variable side chains were synthesized and evaluated in vitro. All ligands showed a favorable lipophilicity, and several were able to outperform the binding affinity of thalidomide as a reference. In addition, most compounds showed low intrinsic cytotoxicity in myeloma cell lines and human peripheral blood mononuclear cells, and did not recruit canonical neosubstrates. A cellular thermal shift assay further demonstrated that the most potent analogues stabilize CRBN in live cells, confirming their on-target engagement. The development of the series was accompanied by a crystallographic study, which rationalizes the observed improvements in binding affinity and neosubstrate selectivity, and can support further development towards molecular glue activity and PROTACs design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


