铜增强水合铁Fenton通过表面亲和分化途径产生双氧化剂降解污染物
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
摘要
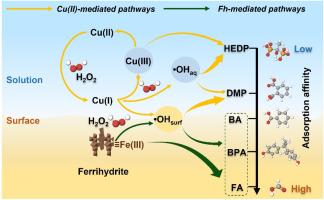
水合铁(Fh)和过渡金属离子(如Cu(II))在自然和工程水生系统中普遍存在,通过类芬顿过程影响污染物的降解。与以往的研究只关注Cu(II)催化的均相反应或氧化铁介导的非均相反应不同,这项工作提出了Cu(II)-Fh在环境相关pH条件下相互作用的综合观点。我们证明Cu(II)和Fh共同建立了一个双氧化系统,能够同时产生羟基自由基(•OH)和Cu(III)。这些反应性物种通过不同的空间机制运作,导致污染物特定的降解行为。研究了甲酸酯(FA)、苯甲酸(BA)、双酚A (BPA)、邻苯二甲酸二甲酯(DMP)和羟乙基二膦酸(HEDP)等5种有机化合物对矿物表面亲和力和活性物质分布的影响。吸附的化合物(FA, BA和BPA)主要通过Fh表面的非自由基内球电子转移进行降解,•OH参与有限。相比之下,弱吸附污染物HEDP的降解主要由•OH和Cu(III)控制,而DMP的吸附可以忽略,Cu(III)的反应活性最小,主要通过吸附表面Cu(II)存在的•OH产生。这种亲和分化的氧化模式强调了污染物结构、配位化学和界面氧化还原动力学如何共同控制矿泉水系统中的降解途径。Cu(II)具有双重催化作用:促进自由基的形成,并通过Cu(III)作为强络合配体的选择性氧化剂。这些发现促进了对cu增强非均相Fenton系统的机制理解,并为氧化还原活性水生环境中污染物的命运和转化提供了新的见解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Copper-Enhanced Ferrihydrite Fenton Generates Dual Oxidants for Pollutant Degradation via Surface Affinity Differentiated Pathways
Ferrihydrite (Fh) and transition metal ions such as Cu(II) are ubiquitous in natural and engineered aquatic systems, influencing pollutant degradation through Fenton-like processes. In contrast to previous studies focusing exclusively on either Cu(II)-catalyzed homogeneous reactions or iron oxide-mediated heterogeneous reactions, this work presents an integrated view of Cu(II)-Fh interactions under environmentally relevant pH conditions. We demonstrate that Cu(II) and Fh jointly establish a dual-oxidant system capable of generating both hydroxyl radicals (•OH) and Cu(III). These reactive species operate through spatially distinct mechanisms, leading to pollutant-specific degradation behaviors. Five organic compounds, including formate (FA), benzoic acid (BA), bisphenol A (BPA), dimethyl phthalate (DMP), and hydroxyethylidene diphosphonic acid (HEDP), were examined to elucidate the roles of mineral surface affinity and reactive species distribution. Adsorbed compounds (FA, BA and BPA) were primarily degraded via nonradical inner-sphere electron transfer at the Fh surface, with limited •OH involvement. In contrast, weakly adsorbing pollutant, HEDP, degradation was governed by •OH and Cu(III), while DMP, which exhibited negligible adsorption and minimal Cu(III) reactivity, proceeded mainly through •OH generated with the presence of adsorbed surface Cu(II). This affinity-differentiated oxidation paradigm highlights how pollutant structure, coordination chemistry, and interfacial redox dynamics jointly control degradation pathways in mineral-water systems. Cu(II) plays a dual catalytic role: enhancing radical formation and acting as a selective oxidant via Cu(III) for strongly complexing ligands. These findings advance the mechanistic understanding of Cu-enhanced heterogeneous Fenton systems and provide new insight into contaminant fate and transformation in redox-active aquatic environments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


