pd催化芳基(伪)卤化物叠氮化反应
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
有机叠氮化物是有机合成、化学生物学和药物发现的有力工具。钯催化剂已经成为芳基亲电试剂形成C-N键的通用工具,但叠氮化物作为一种罕见的以氮为中心的亲核试剂,其合适的催化剂尚未被确定。本文报道了一种以叠氮化钠为催化剂,以溴化芳基和三氟化芳基为原料,pd催化合成叠氮芳基的有效方法。各种杂环,以及与现有方法不相容的芳基亲电试剂(如三氟酸盐),进行有效的叠氮化。实验和计算机制研究指出,体积庞大的辅助膦配体对促进该反应至关重要,具有三种不同的作用。首先,磷化氢的结构降低了LPd(Ar)N3的还原消除障碍;二是减少了非循环Pd2(μ-N3)2二聚体的形成;最后,它提供了LPd(0)对芳基叠氮化物产物失活的抗性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
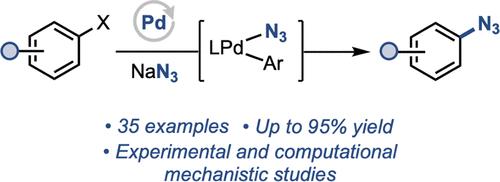
Pd-Catalyzed Azidation of Aryl (Pseudo)Halides
Organic azides are powerful tools in organic synthesis, chemical biology, and drug discovery. Pd catalysts have become general tools for C–N bond formation from aryl electrophiles, but azide stands out as a rare example of a nitrogen-centered nucleophile for which suitable catalysts have not been identified. Herein, we report the development of an effective Pd-catalyzed method for the synthesis of aryl azides from aryl bromides and aryl triflates using sodium azide as a convenient reagent. A variety of heterocycles, as well as aryl electrophiles incompatible with existing approaches (e.g., triflates), undergo efficient azidation. Experimental and computational mechanistic studies point to three distinct roles of the bulky ancillary phosphine ligands critical to facilitating this reaction. First, the structure of the phosphine lowers the barrier to reductive elimination from LPd(Ar)N3; second, it minimizes the formation of off-cycle Pd2(μ-N3)2 dimer; finally, it affords resistance of the LPd(0) toward inactivation by the aryl azide product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


