Kaji-ichigoside F1减轻溃疡性结肠炎的影响:肠道微生物群、代谢组学和转录组学的综合策略。
IF 8.3
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
背景:Kaji-ichigoside F1 (KF1)因其抗菌和抗炎的特性而被广泛认可。然而,其对溃疡性结肠炎(UC)的具体作用及其相关机制尚不清楚。目的:研究KF1减轻UC的作用,并阐明其潜在的药理机制。方法:采用葡聚糖硫酸钠(DSS)诱导UC小鼠模型,再用KF1处理或不处理UC小鼠模型。采用16S rRNA基因测序、代谢组学和转录组学技术探讨KF1对UC的缓解作用。最后,我们阐明了关键功能菌和KF1调控的代谢在UC中的重要作用。结果:KF1干预改善了UC,改变了肠道微生物群的组成,增加了有益细菌普通拟杆菌的丰度。此外,KF1增加了有益代谢途径的水平。值得注意的是,KF1显著抑制炎症因子,改善dss诱导的UC。转录组学分析显示KF1抑制PI3K/AKT信号通路。结论:KF1通过调节肠道菌群和代谢来改善UC,从而在抑制促炎途径的同时创造更有利的状态。本文章由计算机程序翻译,如有差异,请以英文原文为准。
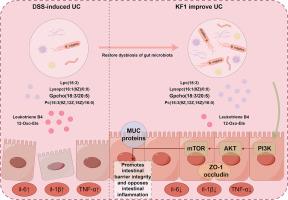
Kaji-ichigoside F1 alleviates the effect of ulcerative colitis: An integrated strategy of gut microbiota, metabolomics, and transcriptomics
Background
Kaji-ichigoside F1 (KF1) is widely recognized for its antibacterial and anti-inflammatory properties. However, its specific effects on ulcerative colitis (UC) and the associated mechanisms remain unclear.
Purpose
We investigated the effect of KF1 in alleviating UC and elucidated its potential pharmacological mechanisms.
Methods
We established a UC mouse model by inducing UC with sodium dextran sulfate (DSS) and then treating or not treating it with KF1. 16S rRNA gene sequencing, metabolomics, and transcriptomics techniques were used to explore the alleviative effect of KF1 on UC. Finally, we elucidated the important roles of key functional bacteria and metabolism regulated by KF1 in UC.
Results
The KF1 intervention ameliorated UC and altered the composition of the gut microbiota, increasing the abundance of the beneficial bacterium Bacteroides vulgatus. Furthermore, KF1 increased the levels of the beneficial metabolic pathway. Notably, KF1 significantly suppressed inflammatory factors and improved DSS-induced UC. Transcriptomic analysis revealed that KF1 inhibited the PI3K/AKT signaling pathway.
Conclusion
KF1 ameliorates UC by modulating the gut microbiota and metabolism to create a more favorable state while inhibiting pro-inflammatory pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Phytomedicine
医学-药学
CiteScore
10.30
自引率
5.10%
发文量
670
审稿时长
91 days
期刊介绍:
Phytomedicine is a therapy-oriented journal that publishes innovative studies on the efficacy, safety, quality, and mechanisms of action of specified plant extracts, phytopharmaceuticals, and their isolated constituents. This includes clinical, pharmacological, pharmacokinetic, and toxicological studies of herbal medicinal products, preparations, and purified compounds with defined and consistent quality, ensuring reproducible pharmacological activity. Founded in 1994, Phytomedicine aims to focus and stimulate research in this field and establish internationally accepted scientific standards for pharmacological studies, proof of clinical efficacy, and safety of phytomedicines.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


