定点固定化d-氨基酸脱氢酶合成d-苯丙氨酸。
IF 4.9
2区 生物学
Q1 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
摘要
芳香族d-氨基酸(d-AAs)作为一种手性组成部分,随着生物催化过程成为其不对称合成的有力方法,越来越受到人们的关注。d-氨基酸脱氢酶(DAADH)是由中二氨基磺酸脱氢酶通过蛋白质工程技术开发出来的,是一种高效的生物催化剂,用于还原胺基生产d-氨基酸。酶固定化允许生物催化剂的回收和再利用,同时也提高了它们的操作稳定性,这对工业应用至关重要。由于对DAADH的固定化研究较少,我们的目标是通过探索其位点特异性的共价固定化,进一步研究热球脲杆菌DAADH的共价固定化。UtDAADH的2、58、92、185、192和317位的几个表面Ser残基被单独替换为Cys,使它们能够固定在马来酰亚胺功能化的Purolite®ECR8415F甲基丙烯酸载体上。通过Cys2和Cys192固定的UtDAADH比活性最高,分别为~0.078和~0.083 U/mg,比共价非特异性固定的UtDAADH高2.1倍和2.2倍。通过10次苯基丙酮酸还原胺化循环对固定化制剂的可回收性进行了测试,根据转化率和比活性的保留情况,S2C固定化的UtDAADH是表现最好的生物催化剂,在第10次循环后,转化率保持在65-70%,活性保持在初始活性的50%。在评估了最佳酶底物比后,对固定化的S2C UtDAADH进行了连续3次200 mg级反应测试,获得了完全转化的对映纯d-Phe,分离率高达88%,支持了其合成适用性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
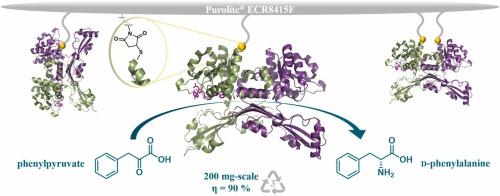
Site-specifically immobilized D-amino acid dehydrogenase for the synthesis of D-phenylalanine
Aromatic d-amino acids (d-AAs) have gained increasing attention as chiral building blocks, with biocatalytic procedures emerging as powerful methods for their asymmetric synthesis. d-Amino acid dehydrogenases (DAADH), developed by protein engineering from meso-diaminopimelate dehydrogenases, step out as highly efficient biocatalysts for the reductive amination-based production of d-AAs. Enzyme immobilization allows the recovery and reuse of biocatalysts, while also enhances their operational stability, which is essential for industrial applications. Since the immobilization of DAADHs have been less explored, we targeted to further progress within the covalent immobilization of DAADH from Ureibacillus thermosphaericus, by exploring its site-specific, covalent immobilization. The individual replacement of several surficial Ser residues to Cys at positions 2, 58, 92, 185, 192 and 317 of UtDAADH, allowed their site-specific immobilization onto the maleimide-functionalized Purolite® ECR8415F methacrylic support. The highest specific activity values, 0.078 and 0.083 U/mg provided by immobilization through Cys2 and Cys192, respectively, showed 2.1- and 2.2-fold higher values compared to the covalently, but non-specifically immobilized UtDAADH. The recyclability of the immobilized preparations was tested among 10 reductive amination-cycles of phenylpyruvate and based on the retained conversion and specific activities, UtDAADH immobilized through S2C was the best-performing biocatalyst, maintaining 65–70 % conversion and 50 % of the initial activity after the 10th cycle. After the assessment of optimal enzyme/substrate ratio, the immobilized S2C UtDAADH was tested in three consecutive 200 mg-scale reaction, providing the enantiopure d-Phe with complete conversions and excellent > 88 % isolation yields, supporting its synthetic applicability.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

New biotechnology
生物-生化研究方法
CiteScore
11.40
自引率
1.90%
发文量
77
审稿时长
1 months
期刊介绍:
New Biotechnology is the official journal of the European Federation of Biotechnology (EFB) and is published bimonthly. It covers both the science of biotechnology and its surrounding political, business and financial milieu. The journal publishes peer-reviewed basic research papers, authoritative reviews, feature articles and opinions in all areas of biotechnology. It reflects the full diversity of current biotechnology science, particularly those advances in research and practice that open opportunities for exploitation of knowledge, commercially or otherwise, together with news, discussion and comment on broader issues of general interest and concern. The outlook is fully international.
The scope of the journal includes the research, industrial and commercial aspects of biotechnology, in areas such as: Healthcare and Pharmaceuticals; Food and Agriculture; Biofuels; Genetic Engineering and Molecular Biology; Genomics and Synthetic Biology; Nanotechnology; Environment and Biodiversity; Biocatalysis; Bioremediation; Process engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


