黄芪多糖增强ogt介导的o - glcn酰化,稳定PINK1诱导d -半乳糖处理的C2C12成肌细胞自噬。
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
背景:黄芪多糖(黄芪多糖)具有缓解肌肉萎缩的作用。本研究探讨了APS对d -半乳糖(D-gal)诱导的C2C12成肌细胞线粒体自噬的影响及其机制。方法:采用CCK-8法测定C2C12成肌细胞的细胞活力。为了进一步阐明APS的作用,我们在C2C12成肌细胞中评估了骨骼肌细胞直径和线粒体自噬,并在有和没有O-GlcNAc转移酶(OGT)的情况下进行了研究。采用肌球蛋白重链(MyHC)免疫荧光染色和western blot分析。通过共免疫沉淀(Co-IP)实验和免疫荧光染色检测OGT与pten诱导的推定激酶1 (PINK1)之间的相互作用。在体内,用D-gal诱导雄性C57BL/6 J小鼠肌肉减少症,并给予APS以评估其对肌肉功能和线粒体健康的影响。结果:黄芪多糖通过OGT诱导o - glcn酰化,促进体外线粒体自噬。OGT敲低显著削弱APS的保护作用。OGT通过S425位点通过o - glcn酰化修饰PINK1。在体内,APS处理显著提高d -gal诱导的肌肉减少症小鼠的握力和肌肉质量。组织学分析显示腓肠肌纤维横截面积增加,Western blot分析显示肌肉组织中LC3II、PINK1、Parkin的表达增强。结论:APS共同促进ogt介导的o - glcn酰化以稳定PINK1,从而促进d -gal处理的C2C12成肌细胞的自噬。在体内,APS可改善肌肉减少症小鼠模型的肌肉功能和线粒体健康。这些发现提示APS可作为一种潜在的治疗肌肉萎缩及相关疾病的药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
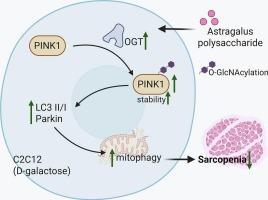
Astragalus polysaccharide enhances OGT-mediated O-GlcNAcylation to stabilize PINK1 to induce mitophagy in D-galactose treated C2C12 myoblasts
Background
Astragalus polysaccharide (APS) has been shown to alleviate muscle atrophy. This study investigated the effects and underlying mechanisms of APS on D-galactose (D-gal)-induced mitochondrial autophagy in C2C12 myoblasts.
Methods
Cell viability in C2C12 myoblasts was assessed using the CCK-8 assay. To further elucidate the role of APS, we evaluated skeletal muscle cell diameter and mitochondrial autophagy in C2C12 myoblasts, with and without O-GlcNAc transferase (OGT). Immunofluorescence staining for myosin heavy chain (MyHC) and western blot analysis were employed. Co-immunoprecipitation (Co-IP) experiments and immunofluorescence staining were conducted to examine the interaction between OGT and PTEN-induced putative kinase 1 (PINK1). In vivo, male C57BL/6 J mice were treated with D-gal to induce sarcopenia, and APS was administered to assess its effects on muscle function and mitochondrial health.
Results
APS promoted mitophagy in vitro by inducing O-GlcNAcylation through OGT. Knockdown of OGT significantly weakened the protective effects of APS. OGT modifies PINK1 with O-GlcNAcylation through the S425 site. In vivo, APS treatment significantly improved grip strength and muscle mass in D-gal-induced sarcopenia mice. Histological analysis showed increased cross-sectional area of gastrocnemius muscle fibers, and Western blot analysis revealed enhanced expression of LC3II, PINK1, and Parkin in muscle tissues.
Conclusion
Collectively, APS promotes OGT-mediated O-GlcNAcylation to stabilize PINK1, thereby facilitating mitophagy in D-gal-treated C2C12 myoblasts in vitro. In vivo, APS improves muscle function and mitochondrial health in a mouse model of sarcopenia. These findings suggest that APS could serve as a potential therapeutic agent for muscle atrophy and related conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


