巨噬细胞内聚苯乙烯纳米塑料的溶酶体依赖胞吞作用。
IF 3.5
3区 医学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
纳米塑料作为新兴污染物已成为具有潜在健康风险的重要环境污染物,由于其独特的物理化学性质和无处不在的环境分布,受到了广泛的研究。然而,对NPs在细胞内完全迁移的研究是有限的,特别是在胞外分泌方面。在这里,我们将人巨噬细胞暴露于聚苯乙烯纳米塑料(PS-NPs)中,观察到PS-NPs诱导细胞内溶酶体的积累并导致其含量增加。此外,PS-NPs与溶酶体共定位并触发溶酶体活化。利用先前建立的PS-NPs吸附细胞内蛋白的方法,并采用蛋白质组学和生物信息学方法,我们证实PS-NPs进入细胞后主要吸附与溶酶体途径相关的蛋白,并稳定吸附溶酶体关键蛋白组织蛋白酶D (CTSD)。进一步的研究发现,PS-NPs诱导溶酶体胞吐,在此过程中,溶酶体特异性成熟的ctsd吸附在PS-NPs上,并从细胞中共释放。这个过程是由Ca2+介导的。综上所述,本研究阐明了PS-NPs的溶酶体依赖胞吞作用,并建立了一种以成熟- ctsd为标记物验证溶酶体胞吐作用的新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
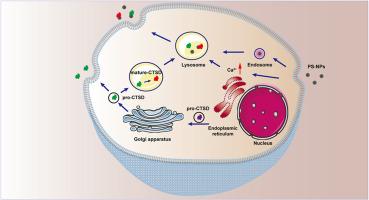
Lysosomal dependent transcytosis of polystyrene nanoplastics within macrophages
As emerging pollutants, nanoplastics (NPs) have emerged as significant environmental pollutants with potential health risks and have been largely investigated owing to their distinctive physicochemical properties and ubiquitous environmental distribution. However, research on the intracellular complete migration of NPs is limited, particularly with respect to exocytosis. Here, we exposed human macrophages to polystyrene nanoplastics (PS-NPs) and observed that PS-NPs induced the accumulation of lysosomes within the cells and lead to an increase in their contents. Additionally, PS-NPs co-localized with lysosomes and triggered lysosomal activation. Using a previously established method for PS-NPs adsorption to intracellular proteins and employing proteomic and bioinformatic approaches, we confirmed that after entering the cell, PS-NPs predominantly adsorbed proteins related to the lysosomal pathway, and stably adsorbed the key lysosomal protein cathepsin D (CTSD). Further studies identified that PS-NPs induced lysosomal exocytosis, during which the lysosomal-specific mature-CTSD adsorbed onto PS-NPs and was co-released from the cell. This process was mediated by Ca2+. In summary, this study elucidated the lysosome-dependent transcytosis of PS-NPs and established a novel method for verifying lysosomal exocytosis using mature-CTSD as a marker.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Food and Chemical Toxicology
工程技术-毒理学
CiteScore
10.90
自引率
4.70%
发文量
651
审稿时长
31 days
期刊介绍:
Food and Chemical Toxicology (FCT), an internationally renowned journal, that publishes original research articles and reviews on toxic effects, in animals and humans, of natural or synthetic chemicals occurring in the human environment with particular emphasis on food, drugs, and chemicals, including agricultural and industrial safety, and consumer product safety. Areas such as safety evaluation of novel foods and ingredients, biotechnologically-derived products, and nanomaterials are included in the scope of the journal. FCT also encourages submission of papers on inter-relationships between nutrition and toxicology and on in vitro techniques, particularly those fostering the 3 Rs.
The principal aim of the journal is to publish high impact, scholarly work and to serve as a multidisciplinary forum for research in toxicology. Papers submitted will be judged on the basis of scientific originality and contribution to the field, quality and subject matter. Studies should address at least one of the following:
-Adverse physiological/biochemical, or pathological changes induced by specific defined substances
-New techniques for assessing potential toxicity, including molecular biology
-Mechanisms underlying toxic phenomena
-Toxicological examinations of specific chemicals or consumer products, both those showing adverse effects and those demonstrating safety, that meet current standards of scientific acceptability.
Authors must clearly and briefly identify what novel toxic effect (s) or toxic mechanism (s) of the chemical are being reported and what their significance is in the abstract. Furthermore, sufficient doses should be included in order to provide information on NOAEL/LOAEL values.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


