1′-Cyanocytidine-5′- isobutyyl在培养和感染的叙利亚仓鼠中是有效的SARS-CoV-2抑制剂
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
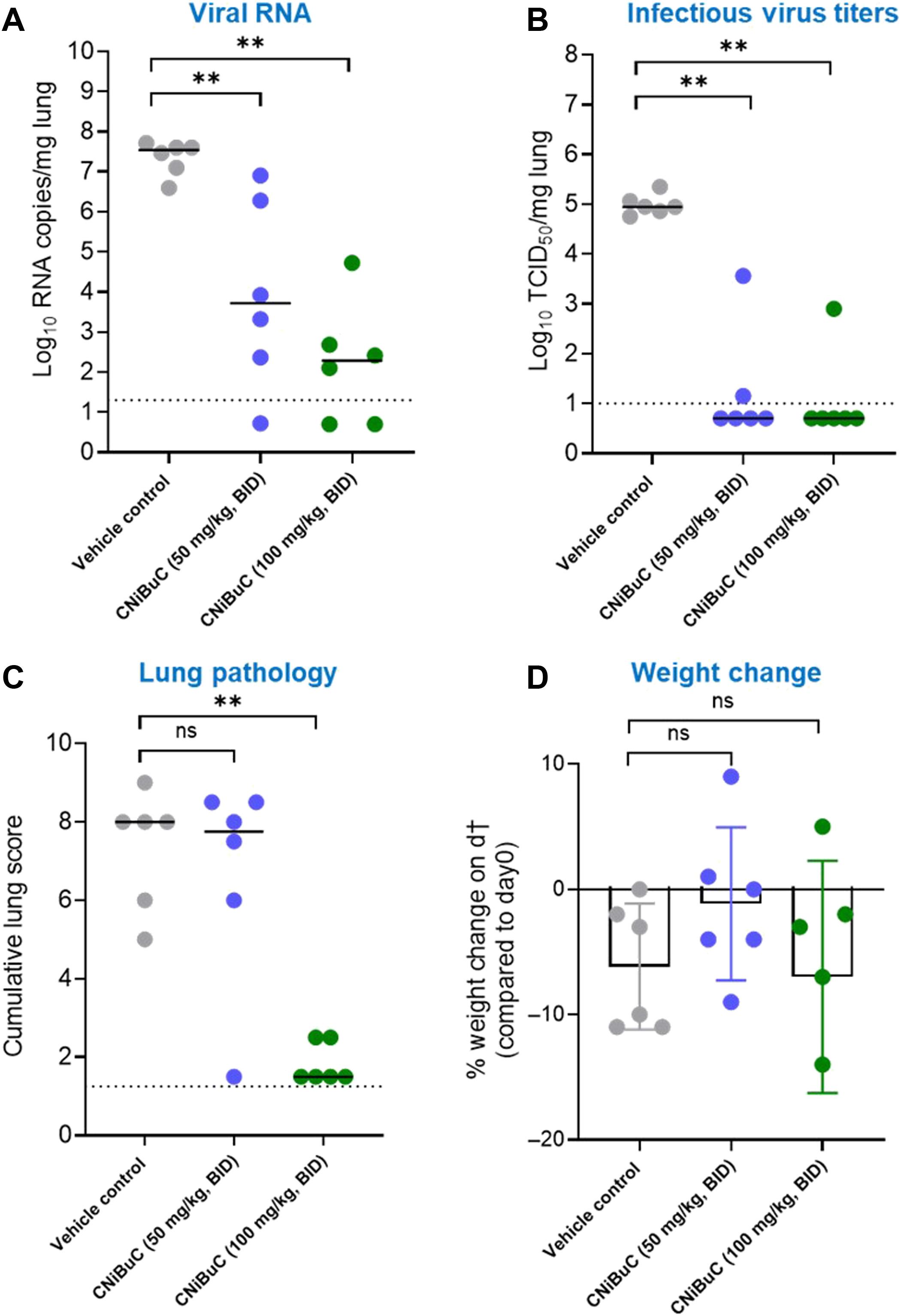
由于疫苗和少量药物的快速开发和部署,严重急性呼吸综合征(SARS-CoV-2)和COVID-19疫情相对得到控制。然而,挑战仍然存在,因为新的变体和疫苗持久性问题可能会损害其有效性。在这里,我们报告了1 ' -氰胞苷(CNC)的发现和评估,这是一种无毒的下一代核苷类似物,在各种细胞和3D HAE-ALI原代培养系统中显示出亚微摩尔抑制SARS-CoV-2复制。在细胞内,CNC被代谢成活性的5 ' -三磷酸形式(CNC- tp),靶向病毒RNA依赖的RNA聚合酶。预稳态动力学分析表明,CNC-TP是一种可逆的竞争性抑制剂。CNC的5 ' -异丁基酯前药(CN iBu C)在小鼠和仓鼠血浆中迅速转化为CNC,经腹腔和口服给药后,在叙利亚仓鼠模型中显著降低病毒RNA水平和肺部感染性病毒滴度。CNC和CN iBu C具有良好的药代动力学、生物利用度和安全性,是SARS-CoV-2治疗的有希望的候选药物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
1′-Cyanocytidine-5′-isobutyryl is a potent SARS-CoV-2 inhibitor in culture and infected Syrian hamsters
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and COVID-19 epidemic is relatively under control due to the rapid development and deployment of vaccines and a few drugs. However, challenges persist, as new variants and issues with vaccine durability may compromise their effectiveness. Here, we report the discovery and evaluation of 1′-cyanocytidine (CNC), a nontoxic, next-generation nucleoside analog that displays submicromolar inhibition of SARS-CoV-2 replication in various cell and 3D HAE-ALI primary culture systems. Intracellularly, CNC is metabolized to its active 5′-triphosphate form (CNC-TP), targeting the viral RNA–dependent RNA polymerase. Pre–steady-state kinetic analysis revealed CNC-TP is a reversible, competitive inhibitor. The 5′-isobutyryl ester prodrug of CNC (CNiBuC), which rapidly converts to CNC in mouse and hamster plasma, substantially reduced viral RNA levels and lung infectious virus titers in a Syrian hamster model after intraperitoneal and oral dosing. With favorable pharmacokinetics, bioavailability, and safety profiles, CNC and CNiBuC represent promising candidates for SARS-CoV-2 therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


