在几秒钟内从天然矿石中一步分离锂
IF 12.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
锂(Li)是密度最低的金属,是大多数电池设计中的最佳元素。随着对锂需求的增加,利用矿石过量酸浸的冶金技术是常见的。然而,这些技术受到二次废物流对环境造成不利影响的多步骤工艺的限制。在这里,我们展示了从初始锂含量仅为4.8%的矿物中提取锂的一步、无酸、无碱工艺。通过在氯化氢(fjh -氯化氢)气氛下对α-锂辉石进行闪焦耳加热,LiCl立即从剩余的非挥发性铝和硅氧化物中蒸馏出来。可以实现纯度为97%,收率为94%的LiCl,大大降低了成本和废物排放。用FJH-Cl 2在当地加工可以显著降低获得锂的复杂性和成本,避免了远程开采,促进了世界向更清洁的可再生能源的发展,这也为从其他矿物中提取关键金属铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
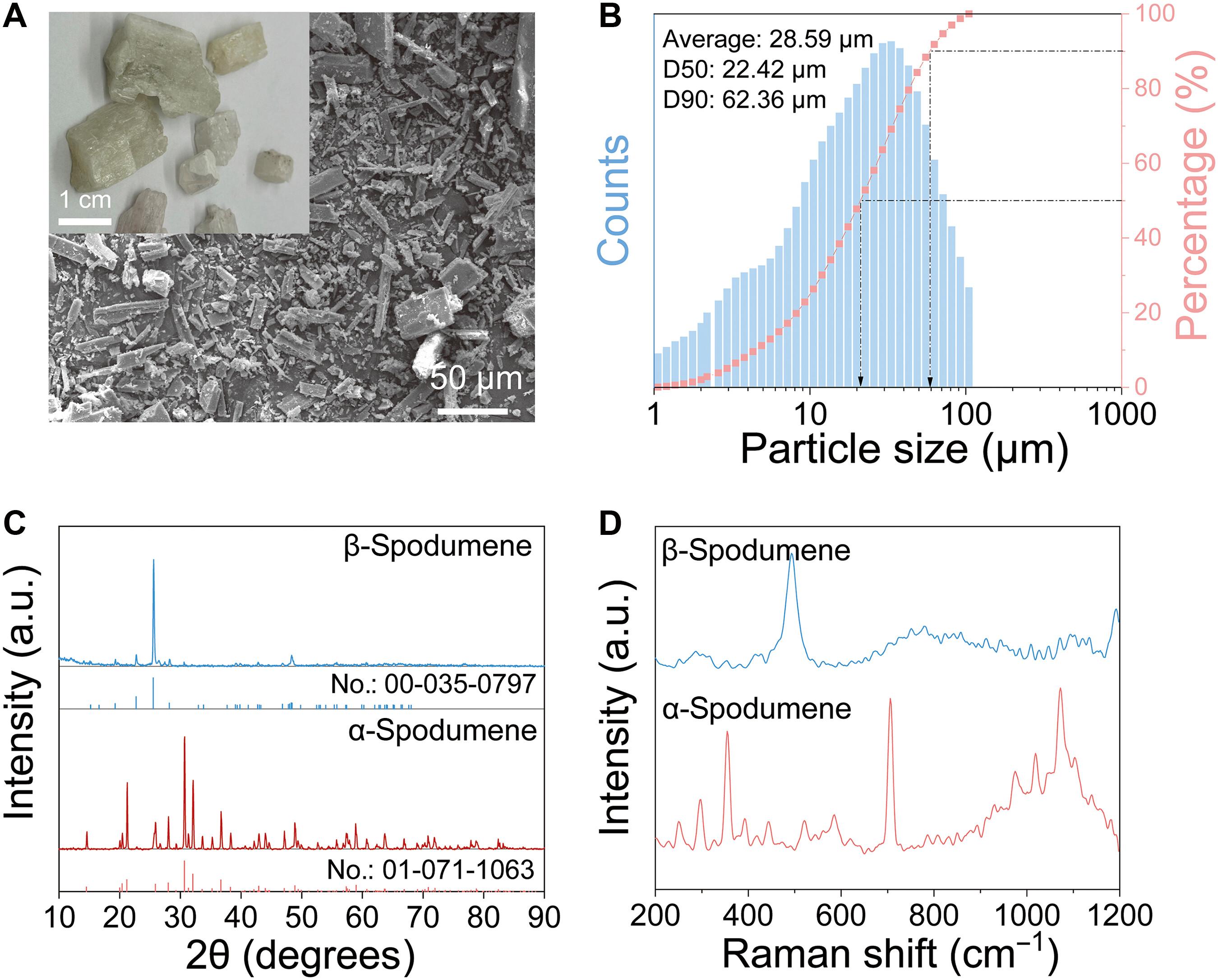
One-step separation of lithium from natural ores in seconds
Lithium (Li), the lowest-density metal, is an optimal element in most battery designs. With the increasing demand for Li, metallurgical techniques using excess acid leaching of mineral ores are common. However, these techniques are limited by multistep processes with adverse environmental impacts caused by secondary waste streams. Here, we show a one-step, acid-free, and alkali-free extraction process for Li from mineral ores with an initial Li content of only 4.8%. By applying flash Joule heating to α-spodumene under an atmosphere of Cl2 (FJH-Cl2), LiCl immediately distills from the remaining nonvolatile aluminum and silicon oxides. LiCl with a 97% purity and 94% yield can be achieved, enormously reducing costs and waste emissions. Local processing with FJH-Cl2 can markedly lessen the complexity and cost of obtaining Li, obviating remote mining and facilitating the world’s progression toward cleaner renewable energies, which also paves the way for extracting critical metals from other mineral ores.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


