基于无背景SERS策略的三维拉曼成像准确灵敏地测定唾液酸
IF 6
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
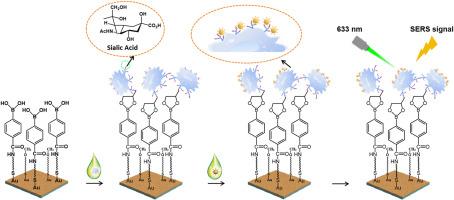
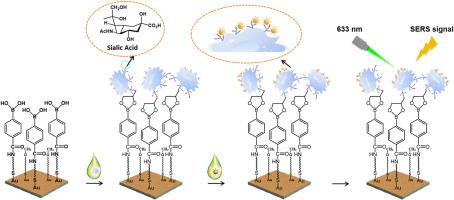
唾液酸(SA)是一种重要的单糖,作为糖蛋白或糖脂的末端部分存在于细胞膜上,与多种生物学和病理活动密切相关,特别是与肿瘤的生长和转移密切相关。众所周知,SA水平的过表达可促进肿瘤免疫逃逸、细胞增殖和血管生成。因此,SA谱分析对于肿瘤的早期识别和长期监测至关重要。然而,它仍然受到目前可用技术的灵敏度和准确性的限制。结果在本文中,我们提出了一种基于信号放大策略的三维(3D)无背景表面增强拉曼散射(SERS)成像方法,以确定单细胞水平上SA的表达。在本研究中,靶细胞首先被苯硼酸(PBA)配体包覆的等离子体金(pAu)薄膜捕获,并由于硼酸键进一步与苯硝基编码的金SERS纳米标签(Au-MBN@Au@PBA)偶联,从而增强等离子体结构,产生各种“热点”,以改善细胞拉曼沉默区(1800-2800 cm-1) 2230 cm-1通道的SERS信号。在这项研究中,3D SERS成像可以深入了解SA水平,确定正常和癌细胞系,并实时监测癌细胞中的纳米颗粒跟踪。在这项工作中,新制备的PBA配体具有多种功能,包括对SERS纳米标签和pAu膜的修饰,对SA基团产生良好的亲和力,以及增加pAu底物的亲水性,用于细胞捕获。在PBA配体的辅助下,获得了pAu膜-细胞- sers纳米标签夹层结构,并产生了增强的等离子体偶联,从而实现了无创、高精度、敏感的SA谱分析,用于早期肿瘤诊断、糖基化探索和吞噬追踪。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Accurate and sensitive determination of sialic acid using three-dimensional Raman imaging based on a background-free SERS strategy
Background
Sialic acid (SA) is a significant monosaccharide presented as the terminal moieties of glycoproteins or glycolipids on the cell membrane and closely correlates with various biological and pathological activities, especially in tumor growth and metastasis. It is well known that the overexpression of SA levels could facilitate tumor immune escape, cell proliferation, and angiogenesis. Thus, SA profiling is critical for the early identification and long-term monitoring of tumors. However, it remains limited by the sensitivity and accuracy of the currently available techniques.
Results
Herein, we proposed a three-dimensional (3D) surface-enhanced Raman scattering (SERS) imaging with a background-free pattern to determine the SA expression at the single-cell level based on a signal amplification strategy. In this study, the target cells were first captured by the phenylboronic acid (PBA) ligands-coated plasmonic gold (pAu) film and further conjugated with benzonitrile-encoded gold SERS nanotags (Au-MBN@Au@PBA) due to the borate bonds, resulting in an enhanced plasmonic structure to generate a wide variety of “hot spots” to improve the SERS signals at 2230 cm−1 channel in the cellular Raman-silent region (1800–2800 cm−1). In this study, the 3D SERS imaging enables a deep insight into SA levels, determination of the normal and cancer cell lines, and real-time monitoring of nanoparticle tracking in cancer cells.
Significance
In this work, the newly prepared PBA ligands possess various capabilities, including modification of SERS nanotags and pAu films, generating good affinity toward SA moieties, and increasing the hydrophilicity of pAu substrate for cell capture. With the assistance of PBA ligands, the pAu film-cell-SERS nanotags sandwich structure was yielded, and the enhanced plasmon coupling was produced, which allows noninvasive, high-precision, and sensitive SA profiling for early tumor diagnosis, glycosylation exploration, and tracking phagocytosis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


