光解作用产生的亚砜基亚硝基烯能够选择性地将氮原子插入到n杂环中
IF 19.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本研究描述了一种光化学方法,通过无光敏剂或添加剂的蓝光照射,从实验稳定的前体生成亚砜基亚硝基。在温和的水条件下,合成的亚砜基亚硝基化合物促进了氮原子与n杂环的化学和位点选择性结合。这种方法将容易获得的吡咯、吲哚和咪唑转化为具有高区域选择性的合成挑战性的嘧啶、喹唑啉和三嗪。力学研究和密度泛函理论计算为观察到的区域选择性的起源提供了见解。该反应与多种氧化敏感官能团相容。它适用于生物活性分子的后期功能化,包括天然产物、氨基酸、c -糖苷、n -核苷和药物。操作简单,官能团耐受性和广泛的底物范围强调了该方法的实用性,并增加了蓝光介导转化的曲目,从而有助于探索新的化学空间,应用于药物化学和药物发现。本文章由计算机程序翻译,如有差异,请以英文原文为准。
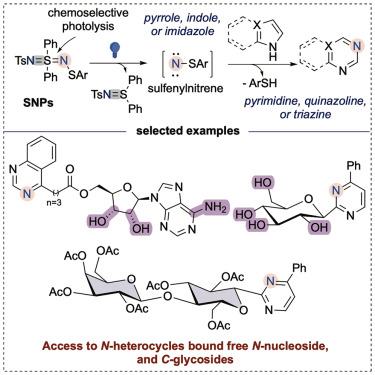
Photolysis-generated sulfenylnitrenes enable site-selective nitrogen-atom insertion into N-heterocycles
This research delineates a photochemical methodology for generating sulfenylnitrenes from bench-stable precursors via blue-light irradiation without photosensitizers or additives. The resultant sulfenylnitrenes facilitate chemo- and site-selective incorporation of nitrogen atoms into N-heterocycles under mild, aqueous conditions. This approach transforms readily accessible pyrroles, indoles, and imidazoles into synthetically challenging pyrimidines, quinazolines, and triazines with high regioselectivity. Mechanistic investigations and density functional theory calculations provide insight into the origins of the observed regioselectivity. The reaction is compatible with a variety of oxidation-sensitive functional groups. It applies to the late-stage functionalization of bioactive molecules, including natural products, amino acids, C-glycosides, N-nucleosides, and pharmaceuticals. The operational simplicity, functional group tolerance, and extensive substrate scope emphasize the utility of this methodology and augment the repertoire of blue-light-mediated transformations, thereby contributing to the exploration of new chemical space for applications in medicinal chemistry and drug discovery.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


