末端烯烃非均相氢甲酰化反应的支链区域选择性:“两步法”及动力学优化
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
烯烃氢甲酰化制醛是化学工业中的一个重要反应。多相催化剂因其易于分离和环境友好而受到广泛关注。尽管研究人员更多地关注线性产物,但支链醛也有广泛的应用,在香料、制药等领域都是重要的化工产品或中间体。在这项工作中,我们提出了一种串联“两步法”方法,即使在使用末端烯烃作为反应物的情况下,也可以在相同的催化剂下获得高区域选择性的支链醛。该方法包括末端烯烃与内烯烃的异构化过程和随后的多相氢甲酰化过程,将内烯烃转化为支化醛。建立了内烯烃非均相氢甲酰化反应的动力学模型,不同反应条件下预测的b/l比与实验结果吻合较好。优化反应条件后,1-辛烯的“″两步法”的最高b/l比达到33.34,比直接氢甲酰化得到的b/l高39倍,并且超过了其他文献报道的所有支链区域选择性。这项工作不仅加深了对端烯烃和内烯烃非均相氢甲酰化反应机理的认识,而且对支化醛的工业化生产具有启发和实践指导意义。本文章由计算机程序翻译,如有差异,请以英文原文为准。
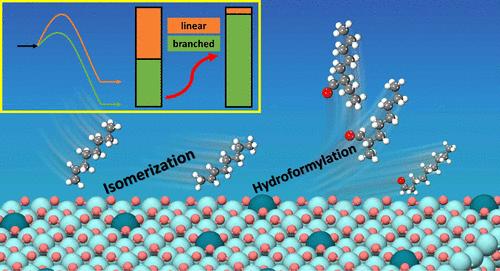
Branched Regioselectivity in the Heterogeneous Hydroformylation of Terminal Olefins: A “Two-Step Process” Method and Kinetic Optimization
Hydroformylation of olefins to aldehydes is an important reaction in the chemical industry. The development of heterogeneous catalysts has attracted much attention due to their ease of separation and environmental friendliness. Although researchers have focused more on linear products, branched aldehydes also have wide applications, serving as important chemical products or intermediates in fragrance, pharmaceutical, and other fields. In this work, we propose a tandem “two-step process” method to obtain high regioselectivity of branched aldehydes with the same catalyst, even when using terminal olefins as the reactants. The method includes the isomerization process of the terminal olefins to internal olefins and the subsequent heterogeneous hydroformylation process, converting internal olefins to branched aldehydes. The kinetic model of heterogeneous hydroformylation of internal olefins is also established, and the predicted b/l ratio under different reaction conditions is consistent with the experimental results. After the optimization of reaction conditions, the highest b/l ratio of the ″two-step process” for 1-octene reaches 33.34, which is 39 times higher than that obtained by direct hydroformylation and surpasses all branched regioselectivities reported in other literature, to the best of our knowledge. This work not only deepens the mechanistic understanding of heterogeneous hydroformylation of both terminal and internal olefins but also provides inspiration and practical guidance for the industrial production of branched aldehydes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


