抗多重耐药恶性疟原虫的新型4-氨基喹啉类抗疟药物Amodiachins的发现
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
为了优化阿莫地喹类似物抗疟药的代谢稳定性和体内活性,进行了构效关系研究。研究了合成的amodiachins (adc)对恶性疟原虫药敏和耐药菌株的抗疟原虫活性。选择的化合物在约利疟原虫小鼠模型中进行了实验。结构修饰,包括引入哌啶环和各种n -烷基取代,增强了抗寄生虫活性、代谢稳定性和体内功效。化合物43 (ADC-028)是一个突出的候选药物,对药物敏感和多重耐药的恶性疟原虫具有纳米摩尔活性,细胞毒性最小,小鼠微粒体稳定性良好(t1/2 = 48.2 min)。在体内,43例达到了16mg /kg/d的非复发剂量和50mg /kg的单次治疗。药代动力学分析显示,43个药物具有良好的代谢稳定性(T1/2 = 84 h)和口服生物利用度(F = 76%)。本研究确定43作为一种新型化合物,作为一种克服耐药疟疾的潜在候选者进行进一步评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
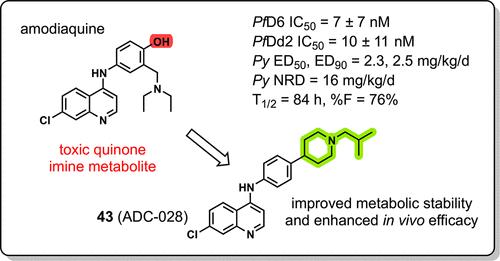
Discovery of Amodiachins, a Novel Class of 4-Aminoquinoline Antimalarials Active against Multidrug-Resistant Plasmodium falciparum
A structure activity relationship study was conducted to optimize metabolic stability and in vivo activity of amodiaquine analog antimalarials. Antiplasmodial activity of synthesized amodiachins (ADCs) were evaluated against drug-sensitive and drug-resistant strains of Plasmodium falciparum. Select compounds were tested in Plasmodium yoelli murine models. Structural modifications, including introduction of a piperidine ring and varied N-alkyl substitutions enhanced antiparasitic activity, metabolic stability, and in vivo efficacy. Compound 43 (ADC-028) emerged as a standout candidate, exhibiting nanomolar activity against drug-sensitive and multidrug-resistant P. falciparum, minimal cytotoxicity, and favorable murine microsomal stability (t1/2 = 48.2 min). In vivo, 43 achieved a nonrecrudescence dose at 16 mg/kg/d and a single dose cure at 50 mg/kg. Pharmacokinetic analysis of 43 showed excellent metabolic stability (T1/2 = 84 h) and oral bioavailability (F = 76%). This study identifies 43 as a potential candidate for further evaluation as a novel compound to overcome drug-resistant malaria.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


