Ni-Al2O3催化剂的拓扑变换合成及其构效关系研究
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
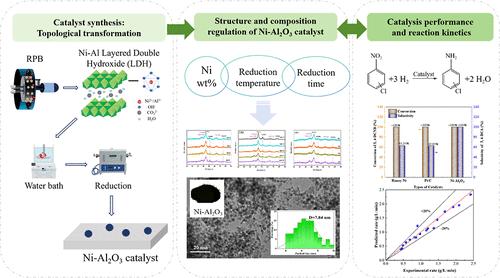
硝基氢化是工业上常见的过程。在旋转填充床(RPB)上合成了一种由镍铝层状双氧根衍生的高效Ni-Al2O3催化剂,首次应用于3,4-二氯硝基苯(3,4- dcnb)加氢成3,4-二氯苯胺(3,4- dca)。利用RPB增强的传质和微混合性能,有效缓解了催化剂合成过程中出现的颗粒团聚和粒度分布不均匀现象。考察了Ni质量分数、还原温度和还原时间对催化剂物理性能和催化性能的影响。结果表明,在Ni质量分数为78%、还原温度为500℃、还原时间为4 h的最佳条件下制备的Ni - al2o3催化剂具有粒径小、分散性好等特点。在优化条件下(65℃,1.5 MPa H2, 60 min,催化剂负载12.55 mg/g)加氢3,4- dcnb,转化率和3,4- dca选择性均接近100%。对3,4- dcnb加氢反应的动力学研究表明,该反应的活化能为39.91 kJ/mol,指前因子为2.54 × 104 min-1。这一发现为制备3,4- dcnb加氢成3,4- dca的高效镍基催化剂提供了一种创新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Study on the Synthesis of Ni–Al2O3 Catalysts via Topological Transformation and Their Structure–Activity Relationship
Nitrohydrogenation is a common process in industry. Here, a highly efficient Ni–Al2O3 catalyst derived from Ni–Al layered double hydroxide synthesized in a rotating packed bed (RPB) was first applied in the hydrogenation of 3,4-dichloronitrobenzene (3,4-DCNB) to 3,4-dichloroaniline (3,4-DCA). Leveraging the enhanced mass transfer and micromixing performances by applying RPB, the phenomenon of particle agglomeration and uneven size distribution occurring during catalyst synthesis has been effectively mitigated. The effects of Ni mass fraction, reduction temperature, and time on the physical properties and catalytic performance of the catalyst were investigated. Results showed that the Ni–Al2O3 catalysts prepared under optimal conditions (Ni mass fraction of 78 wt %, reduction temperature of 500 °C, reduction time of 4 h) have small Ni particle sizes and high dispersion. When applied to 3,4-DCNB hydrogenation under optimized conditions (65 °C, 1.5 MPa of H2, 60 min, catalyst loading 12.55 mg/g), both conversion and 3,4-DCA selectivity approached 100%. Furthermore, the kinetic study on the 3,4-DCNB hydrogenation revealed that the reaction has an activation energy of 39.91 kJ/mol and a pre-exponential factor of 2.54 × 104 min–1. This finding presents an innovative approach for preparing a highly efficient Ni-based catalyst for the hydrogenation of 3,4-DCNB to 3,4-DCA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


