聚醚醚酮植入物表面孔隙结构调节巨噬细胞M2极化的力学性能
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
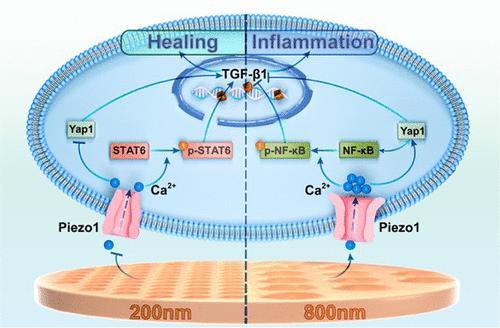
聚醚醚酮(PEEK)表面的微纳米结构可以提高生物材料的生物相容性,促进巨噬细胞M2极化诱导软组织整合。然而,由于PEEK表面难以获得均匀可控的微纳米结构,巨噬细胞M2极化的调控规律和潜在机制尚不清楚。在这项研究中,我们使用热压技术在PEEK材料上制备了尺寸为200、500和800 nm的均匀亚微米多孔结构。这些结构可以显著提高界面亲水性,降低材料的刚度。此外,我们通过细胞实验研究了巨噬细胞M2极化的最佳亚微米结构尺寸,发现PEEK上200 nm的孔可以显著促进巨噬细胞向修复型M2极化,同时伴随着TGF-β1和Arg1等细胞因子的分泌增加。间接共培养实验进一步证实,这些极化巨噬细胞增强了血管内皮细胞和成纤维细胞的增殖和迁移。转录组学分析和分子生物学实验显示,200 nm多孔界面可导致巨噬细胞M2极化过程中Piezo1、Yap1、NF-κB下调,STAT6、TGF-β1上调。此外,C57小鼠实验显示,具有200 nm孔隙的PEEK植入物周围的软组织整合得到改善,并伴有更好的血管化和纤维化。本研究强调,200 nm多孔PEEK植入物可调节巨噬细胞M2极化,促进软组织整合,这一过程依赖于Piezo1/TGF-β1信号通路的激活。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Surficial Pore Structure on Polyetheretherketone Implants Regulates Mechanical Properties to Promote Macrophage M2 Polarization
The surficial micro–nanostructure on polyetheretherketone (PEEK) can increase the biocompatibility of biomaterials and promote macrophage M2 polarization to induce soft tissue integration. However, because it is difficult to obtain a uniform and controllable surficial micro–nanostructure on PEEK, the regulation rules and underlying mechanisms for macrophage M2 polarization remain poorly understood. In this study, we used a hot pressing technique to fabricate uniform submicrometer porous structures with sizes of 200, 500, and 800 nm on PEEK material. These structures can significantly increase the hydrophilicity of the interface and decrease the stiffness of the materials. Furthermore, a cellular experiment was performed to investigate the optimal size of the submicrometer structure for macrophage M2 polarization, and 200 nm pores on PEEK can significantly promote macrophage polarization toward the reparative M2 phenotype, accompanied by increased secretion of cytokines such as TGF-β1 and Arg1. Indirect coculture assays further confirmed that these polarized macrophages enhanced the proliferation and migration of vascular endothelial cells and fibroblasts. Transcriptomic analysis and molecular biology experiments revealed that the 200 nm porous interface can lead to downregulation of Piezo1, Yap1, and NF-κB and upregulation of STAT6 and TGF-β1 in the process of macrophage M2 polarization. Moreover, the C57 mouse experiment showed the improved soft tissue integration surrounding the PEEK implants with 200 nm pores, accompanied by better vascularization and fibrosis. This study highlights that 200 nm porous PEEK implants can modulate macrophage M2 polarization to promote soft tissue integration, and this process relies on the activation of the Piezo1/TGF-β1 signaling pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


