雾化吸入工程细胞外囊泡破坏转移前生态位并抑制小鼠术后肺转移模型
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
远处器官的转移前生态位(PMN)为循环肿瘤细胞(ctc)的定植提供了合适的土壤。靶向PMN破坏正成为对抗肿瘤转移的有效策略。考虑到肺是黑色素瘤转移发生率最高的器官,雾化吸入可以直接将药物输送到肺。本研究构建了M1巨噬细胞来源、过表达cxcr4和装载bms202的细胞外囊泡(BMS@C-M1 EV),以抑制黑色素瘤术后肺转移。经雾化吸入,BMS@C-M1 EV在黑色素瘤术后小鼠肺内有效积累,其表面CXCR4可通过消耗CXCL12抑制单核细胞髓源性抑制细胞(mo-MDSCs)的募集,其M1促炎特性使肿瘤相关巨噬细胞(tam)从M2促肿瘤表型重极化为M1抗肿瘤表型,从而逆转免疫抑制微环境,激活T细胞免疫应答。防止PMN的生成。此外,BMS@C-M1 EV释放的BMS202可诱导CTCs中PD-L1的二聚化,阻断PD-1/PD-L1的相互作用,从而增强T细胞介导的CTCs免疫消除,进一步抑制转移的发生。因此,BMS@C-M1 EV通过雾化吸入可破坏PMN的形成,消除肺内ctc,有效抑制黑色素瘤术后肺转移。这种治疗方法在预防黑色素瘤术后肺转移方面具有很大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
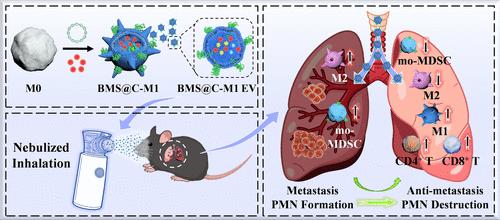
Nebulized Inhalation of Engineered Extracellular Vesicles to Destruct Pre-metastatic Niche and Inhibit Postoperative Lung Metastasis in a Mouse Model
Pre-metastatic niche (PMN) in the distant organs provides a suitable soil for the colonization of circulating tumor cells (CTCs). Targeting PMN destruction is becoming an effective strategy against tumor metastasis. Considering that the lung is the organ with the highest incidence of melanoma metastasis, nebulized inhalation can directly deliver drugs to the lung. Herein, M1 macrophage-derived, CXCR4-overexpressed, and BMS202-loaded extracellular vesicles (BMS@C-M1 EV) were constructed to inhibit postoperative melanoma lung metastasis. After nebulized inhalation, BMS@C-M1 EV effectively accumulated in the lungs of postoperative melanoma mice, its surface CXCR4 could inhibit the recruitment of monocytic myeloid-derived suppressor cells (mo-MDSCs) by consuming CXCL12, and its M1 pro-inflammatory feature repolarized tumor-associated macrophages (TAMs) from the M2 pro-tumor phenotype into the M1 antitumor phenotype, thereby reversing the immunosuppressive microenvironment, activating the T cell immune response, and preventing PMN construction. Furthermore, BMS202 released by BMS@C-M1 EV could induce the dimerization of PD-L1 in CTCs to block the PD-1/PD-L1 interaction, thereby enhancing T cell-mediated immune elimination of CTCs and further inhibiting the occurrence of metastasis. Therefore, BMS@C-M1 EV through nebulized inhalation could disrupt PMN formation and eliminate CTCs in the lung, effectively suppressing postoperative melanoma lung metastasis. This therapeutic approach holds great potential for preventing postoperative melanoma lung metastasis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


