多价金属核苷酸纳米激动剂增强肿瘤金属免疫治疗的先天免疫激活
IF 9.1
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
金属免疫疗法通过利用金属离子的免疫调节功能,单独或与激动剂联合使用,代表了癌症免疫治疗的新范式。然而,游离金属离子和激动剂的低生物利用度限制了抗肿瘤免疫反应的强大刺激。本研究通过将多种基于核苷酸的先天免疫激动剂与Fe2+和Mn2+水配位,开发出多价金属-核苷酸纳米激动剂(Mn-MNAs),不仅增强了激动剂的稳定性和细胞摄取,而且通过同时激活干扰素基因刺激因子(STING)和toll样受体9 (TLR9)途径,增强了抗肿瘤免疫。在CT26肿瘤模型中,瘤内注射Mn-MNAs可显著抑制肿瘤生长并培养免疫支持的肿瘤微环境。静脉给药Mn-MNAs在B16F10黑色素瘤模型中获得了显著的治疗效果,并在联合检查点阻断后进一步增强。总之,多价纳米激动剂为癌症金属免疫治疗开辟了新的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
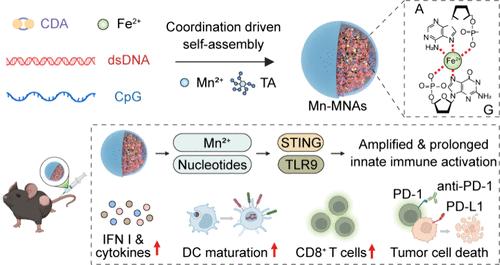
Multivalent Metal–Nucleotide Nanoagonists Amplify Innate Immune Activation for Cancer Metalloimmunotherapy
Metalloimmunotherapy represents a new paradigm in cancer immunotherapy by leveraging the immune-modulatory functions of metal ions, either alone or in combination with agonists. However, the low bioavailability of free metal ions and agonists limits robust stimulation of antitumor immune responses. Here we developed multivalent metal–nucleotide nanoagonists (Mn-MNAs) through aqueous coordination of multiple nucleotide-based innate immune agonists with Fe2+ and Mn2+, which not only enhanced the stability and cellular uptake of the agonists but also amplified the antitumor immunity by concurrently activating the stimulator of interferon genes (STING) and toll-like receptor 9 (TLR9) pathways. Intratumoral injection of Mn-MNAs significantly inhibited tumor growth and fostered an immune-supportive tumor microenvironment in the CT26 tumor model. Intravenous administration of Mn-MNAs achieved remarkable therapeutic efficacy in the B16F10 melanoma model, which was further enhanced upon combination with a checkpoint blockade. Overall, multivalent nanoagonists open new avenues for cancer metalloimmunotherapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nano Letters
工程技术-材料科学:综合
CiteScore
16.80
自引率
2.80%
发文量
1182
审稿时长
1.4 months
期刊介绍:
Nano Letters serves as a dynamic platform for promptly disseminating original results in fundamental, applied, and emerging research across all facets of nanoscience and nanotechnology. A pivotal criterion for inclusion within Nano Letters is the convergence of at least two different areas or disciplines, ensuring a rich interdisciplinary scope. The journal is dedicated to fostering exploration in diverse areas, including:
- Experimental and theoretical findings on physical, chemical, and biological phenomena at the nanoscale
- Synthesis, characterization, and processing of organic, inorganic, polymer, and hybrid nanomaterials through physical, chemical, and biological methodologies
- Modeling and simulation of synthetic, assembly, and interaction processes
- Realization of integrated nanostructures and nano-engineered devices exhibiting advanced performance
- Applications of nanoscale materials in living and environmental systems
Nano Letters is committed to advancing and showcasing groundbreaking research that intersects various domains, fostering innovation and collaboration in the ever-evolving field of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


