手性和荧光硫化铜纳米颗粒的双重信号促进肿瘤细胞自噬
IF 19
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
自噬是多种人类疾病的关键调节因子。本文制备了经青霉胺修饰的手性硫化铜纳米粒子(NPs),该纳米粒子具有300 ~ 1000 nm的圆二色光谱和680 nm的荧光峰。实验数据显示,D-CuS NPs表现出手性依赖性的自噬诱导,优于L-CuS NPs,靶向乳腺癌细胞,对CD81受体的亲和力高228.4倍。其机制揭示了手性NPs特异性结合CD81受体打开机械敏感钙通道,激活钙调素依赖性蛋白激酶激酶β (CaMKKβ)/AMPK通路,从而增强Atg1/ unc -51样自噬激活激酶1 (ULK1)活性,促进自噬。体内实验证实D-CuS NPs在肿瘤中选择性积累,诱导自噬,成功治疗肿瘤。因此,手性CuS NPs在调节生物过程和改善肿瘤治疗方面显示出巨大的潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
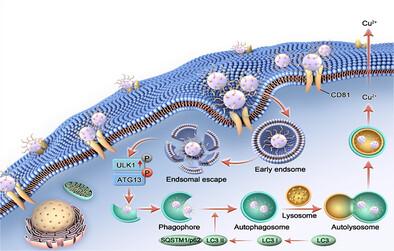
Dual Signals of Chiral and Fluorescent Copper Sulphide Nanoparticles Boost Tumor Cell Autophagy
Autophagy is a key regulator in a diverse range of human diseases. Here, chiral copper sulfide (CuS) nanoparticles (NPs) modified with penicillamine are fabricated, which exhibited a circular dichroism (CD) spectrum between 300 and 1000 nm and a fluorescence peak at 680 nm. The experimental data showed that D-CuS NPs exhibited chirality-dependent autophagy induction, outperforming L-CuS NPs, and targeted breast cancer cells with 228.4 times higher affinity for CD81 receptor. The mechanism is revealed that chiral NPs specifically bound to CD81 receptor to open mechanosensitive calcium channels and motivated the calmodulin-dependent protein kinase kinase β (CaMKKβ)/AMPK pathway, which enhanced Atg1/Unc-51-like autophagy activating kinase 1 (ULK1) activity to promote autophagy. In vivo experiments confirmed the selective accumulation of D-CuS NPs in tumors, induction of autophagy, and successful treatment of the tumor. Therefore, chiral CuS NPs show great potential for modulating biological processes and improving tumor treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


