QS-21:更安全的疫苗佐剂的结构见解、免疫机制和合理设计的前景
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
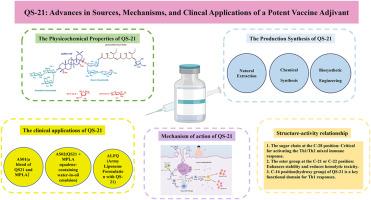
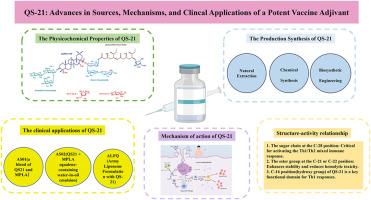
QS-21是一种基于皂苷的精制佐剂,是从皂皮树(Quillaja saponaria)的树皮中分离出来的。由于其强大的诱导th1型和th2型免疫反应的能力,它已经获得了创造下一代疫苗佐剂的重大兴趣。尽管已成功地将其纳入许可疫苗中,如Shingrix®(带状疱疹)和moquirix®(疟疾),但QS-21的广泛应用仍然受到一些限制,包括有限的自然可用性、化学不稳定性、剂量依赖性毒性和不完全阐明的作用机制。本文从体外和体内研究的角度,综述了QS-21的天然来源、分子结构、临床应用和免疫机制。本文还介绍了QS-21的构效关系(SAR)的新进展,强调了通过化学合成和生物合成工程创造危害更小、更稳定的类似物的可能性。这些策略为合理设计新型佐剂以支持开发更有效和更安全的疫苗提供了有希望的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
QS-21: Structural insights, immunological mechanisms, and prospects for rational design of safer vaccine adjuvants
QS-21, a refined adjuvant based on saponins, is isolated from the bark of the soap-bark tree (Quillaja saponaria). It has garnered significant interest in the creation of next-generation vaccine adjuvants due to its robust ability to elicit Th1-type and Th2-type immunological responses. Despite its successful incorporation into licensed vaccines such as Shingrix® (herpes zoster) and Mosquirix® (malaria), the widespread application of QS-21 remains constrained by several limitations, including limited natural availability, chemical instability, dose-dependent toxicity, and an incompletely elucidated mechanism of action. This review provides a comprehensive overview of the natural sources, molecular structure, clinical applications, and immunological mechanisms of QS-21, based on research conducted both in vivo and in vitro. It also presents new developments in QS-21's structure-activity relationship (SAR), highlighting the possibility of creating less hazardous and more stable analogs through chemical synthesis and biosynthetic engineering. These strategies offer promising avenues for the rational design of novel adjuvants to support the development of more effective and safer vaccines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


