CBX4乙酰化作为HIF-1α活性的抑制机制
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
HIF-1α的转录活性通过CBX4介导的SUMOylation而增强。尽管CBX4-HIF-1α轴的重要性已得到公认,但其调控的分子机制仍不清楚。在这项研究中,对101,254个化合物文库进行表型筛选,然后进行结构优化,最终鉴定出XZA-1,这是一种能够破坏cbx4介导的HIF-1α转录激活的小分子。机制研究表明,XZA-1激活脂肪酸β氧化的关键酶HADH,导致细胞内乙酰辅酶a水平升高。该代谢物促进CBX4赖氨酸106的乙酰化,从而降低其SUMO E3连接酶活性。在过表达CBX4的异种移植物模型中,XZA-1通过增强CBX4 K106乙酰化表现出抗肿瘤作用。此外,CBX4 K106乙酰乙酰化水平升高在总生存率较高的临床HCC患者的组织中被观察到。这些发现表明乙酰乙酰辅酶a作为一种潜在的抗肿瘤代谢物,并为调节癌症中HIF-1α转录活性建立了一种新的药理学途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
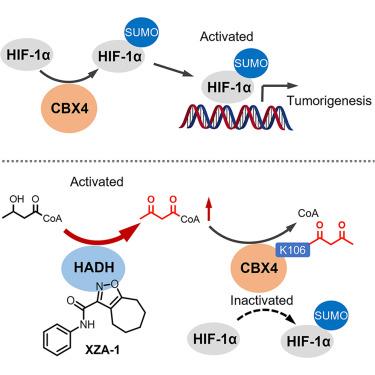
CBX4 acetoacetylation as an inhibitory mechanism of HIF-1α activity
HIF-1α transcriptional activity is enhanced through SUMOylation mediated by CBX4. Despite the recognized importance of the CBX4-HIF-1α axis, the molecular mechanisms governing its regulation remain largely unclear. In this study, phenotypic screening of a 101,254-compound library followed by structural optimization led to the identification of XZA-1, a small molecule capable of disrupting CBX4-mediated HIF-1α transcriptional activation. Mechanistic investigations revealed that XZA-1 activates HADH, a key enzyme in fatty acid β-oxidation, resulting in increased intracellular levels of acetoacetyl-CoA. This metabolite promotes acetoacetylation of CBX4 at lysine 106, thereby reducing its SUMO E3 ligase activity. In a CBX4-overexpressing xenograft model, XZA-1 demonstrated antitumor effects by enhancing CBX4 K106 acetoacetylation. Additionally, elevated levels of CBX4 K106 acetoacetylation were observed in clinical HCC tissues from patients with better overall survival. These findings suggest that acetoacetyl-CoA functions as a potential antitumor metabolite and establish a novel pharmacological approach for modulating HIF-1α transcriptional activity in cancer.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


