聚苯乙烯纳米塑料和草甘膦共同暴露通过肠道屏障损伤和免疫炎症失调促进小鼠肠道凋亡
IF 7.3
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
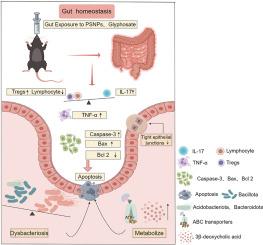
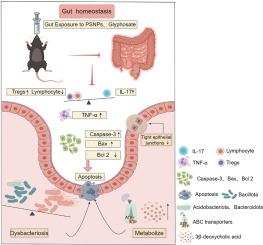
聚苯乙烯纳米塑料(PSNPs, 1-1000 nm)和草甘膦等环境污染物构成重大的环境和公共健康风险。本研究旨在探讨psnp和/或草甘膦诱导的肠道毒性及其分子机制。小鼠通过灌胃(psnp: 0.5 mg/d;草甘膦:50 mg/kg-bw/天;psnp +草甘膦:0.5 mg/d +50 mg/kg-bw/天)暴露于psnp (100 nm)、草甘膦或两者的组合中35天。对照组给予等量蒸馏水。我们的研究结果显示,暴露于psnp和/或草甘膦会加重病理改变,包括炎症细胞浸润,严重的线粒体嵴断裂,以及肠道中杯状细胞减少约50%。此外,暴露于psnp和/或草甘膦导致75%的FOXP3抑制和肠内紧密连接解离(反映在Occludin减少50%,ZO-1减少20%-50%)。这些变化伴随着有益肠道菌群、代谢谱、胆汁酸代谢紊乱的显著改变,以及3-β-去氧胆酸(一种与胆汁酸受体信号传导和屏障功能障碍相关的代谢物)显著升高。虽然暴露于草甘膦会导致促炎因子TNF-α和促凋亡蛋白cleaved -caspase-3的显著上调,但在动物组织实验中,共暴露并未加剧细胞凋亡,这与基于细胞的研究结果相反。小鼠肠上皮细胞用psnp (0.75 mg/mL)或草甘膦(0.5 mg/mL)处理。体外实验表明,psnp加重了草甘膦引起的Treg/Th17免疫-炎症平衡被破坏,肠道屏障功能受损(ZO-1和Occludin减少50%),并增加细胞凋亡。这项研究促进了我们对内分泌干扰化学混合物所带来的健康风险的理解,并为psnp -草甘膦诱导肠道毒性的分子机制提供了重要见解。这些发现为未来旨在减轻环境污染物的病理生理影响的研究奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Co-exposure to polystyrene nanoplastics and glyphosate promotes intestinal apoptosis in mice via intestinal barrier impairment and immunoinflammatory dysregulation
Environmental contaminants such as polystyrene nanoplastics (PSNPs, 1–1000 nm) and glyphosate pose significant environmental and public health risks. This study aimed to investigate the intestinal toxicity and molecular mechanisms induced by PSNPs and/or glyphosate. Mice were exposed to PSNPs (<100 nm), glyphosate, or a combination of both for 35 days via intragastric administration (PSNPs: 0.5 mg/d; glyphosate: 50 mg/kg-bw/day; PSNPs + glyphosate: 0.5 mg/d +50 mg/kg-bw/day). The control group received same volume of distilled water. Our findings revealed that exposure to PSNPs and/or glyphosate aggravated pathological alterations, including inflammatory cell infiltration, severe mitochondrial cristae fracture, and an approximately 50 % reduction in goblet cells in the intestine. Moreover, exposure to PSNPs and/or glyphosate caused a critical 75 % inhibition of FOXP3 and dissociation of tight junctions in the intestine (reflected by a 50 % decrease in Occludin, and a 20 %–50 % decrease in ZO-1). These changes were accompanied by significant alterations in beneficial gut microbiota, metabolic profiles, bile acid metabolism disorders, and a pronounced elevation in 3-β-deoxycholic acid, a metabolite tied to bile acid receptor signaling and barrier dysfunction. Although exposure to glyphosate led to the most significant upregulation of the pro-inflammatory factors TNF-α and the pro-apoptosis proteins Cleave-caspase-3, co-exposure did not exacerbate cell apoptosis in animal tissue experiments, which is contrasts with the cell-based findings. MODE-K (mouse intestinal epithelial) cells were treated with PSNPs (0.75 mg/mL) or glyphosate (0.5 mg/mL). In vitro experiments showed that PSNPs aggravated the disrupted Treg/Th17 immune-inflammatory balance, impaired intestinal barrier function (with a 50 % reduction in ZO-1 and Occludin), and increased cell apoptosis, caused by glyphosate. This study advances our understanding of the health risks posed by endocrine-disrupting chemical mixtures and provides critical insights into the molecular mechanisms of PSNP-glyphosate-induced intestinal toxicity. These findings lay the groundwork for future research aimed at mitigating the pathophysiological impacts of environmental pollutants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Environmental Pollution
环境科学-环境科学
CiteScore
16.00
自引率
6.70%
发文量
2082
审稿时长
2.9 months
期刊介绍:
Environmental Pollution is an international peer-reviewed journal that publishes high-quality research papers and review articles covering all aspects of environmental pollution and its impacts on ecosystems and human health.
Subject areas include, but are not limited to:
• Sources and occurrences of pollutants that are clearly defined and measured in environmental compartments, food and food-related items, and human bodies;
• Interlinks between contaminant exposure and biological, ecological, and human health effects, including those of climate change;
• Contaminants of emerging concerns (including but not limited to antibiotic resistant microorganisms or genes, microplastics/nanoplastics, electronic wastes, light, and noise) and/or their biological, ecological, or human health effects;
• Laboratory and field studies on the remediation/mitigation of environmental pollution via new techniques and with clear links to biological, ecological, or human health effects;
• Modeling of pollution processes, patterns, or trends that is of clear environmental and/or human health interest;
• New techniques that measure and examine environmental occurrences, transport, behavior, and effects of pollutants within the environment or the laboratory, provided that they can be clearly used to address problems within regional or global environmental compartments.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


