TiO2/InVO4可见光降解利福平催化剂的制备。
IF 8.1
2区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
摘要
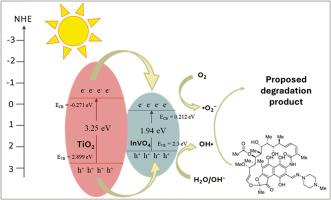
以芒果叶提取物为原料,采用绿色合成法,用InVO4修饰TiO2纳米粒子。芒果叶提取物的水组分中含有次生代谢物,是弱碱和封盖剂的来源。采用FTIR、UV-Vis DRS、XRD、FESEM、EDX、PSA和HRTEM对TiO2纳米颗粒和TiO2/ TiO2纳米复合材料进行了表征。该纳米复合材料的带隙减小到2.70 eV,能够吸收可见光。HRTEM结果显示,该材料的初始粒径在55 ~ 80 nm之间,具有明显的晶格条纹,对应于锐钛矿型TiO2的(101)面和TiO2/InVO4复合材料的(122)面,为异质结的形成提供了直接证据。采用紫外可见分光光度计检测TiO2/InVO4纳米复合材料在可见光下对利福平的光催化活性。与单独的TiO2和InVO4纳米颗粒相比,TiO2/InVO4纳米复合材料具有更好的光催化性能,在120 min内降解效率达到97.18%。动力学研究表明,TiO2/InVO4光催化降解利福平的反应为准一级反应,R2值较高,反应速率常数k为9.34 × 10-3。自由基清除剂测试确定了参与降解过程的主要活性物质,表明空穴(h+)是最重要的,其次是羟基(•OH)和超氧化物(•O2-)自由基。该材料还表现出稳定性和可重复使用性,在连续第五次循环后仍保持其初始活性的84.65%。光催化活性的提升可能归因于与纯TiO2相比,带隙减小,从而能够吸收可见光。利福平是一种严重威胁水生生态系统和人类健康的持久性有机污染物,本研究通过开发一种高效光催化剂来降解利福平,解决了药物污染的紧迫问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Fabrication of TiO2/InVO4 as an effective photocatalyst under visible light for degradation of rifampicin
TiO2 nanoparticles were modified with InVO4 via the green synthesis method using mango (Mangifera indica L.) leaf extract. The water fraction of mango leaf extract consisted of secondary metabolites, which act as sources of weak bases and capping agents. TiO2 nanoparticles and TiO2/InVO4 nanocomposites were characterized using FTIR, UV–Vis DRS, XRD, FESEM, EDX, PSA and HRTEM. The nanocomposite exhibited a reduced band gap of 2.70 eV, enabling visible light absorption. HRTEM, which revealed a primary particle size ranging from 55 to 80 nm and confirmed the crystalline nature of the material, showing distinct lattice fringes corresponding to the (101) plane of anatase TiO2 and the (122) plane of the TiO2/InVO4 composite, providing direct evidence for the formation of a crystalline heterojunction. The photocatalytic activity of TiO2/InVO4 nanocomposites against rifampicin under visible light irradiation was probed by a UV–Vis spectrophotometer. The TiO2/InVO4 nanocomposite exhibited superior photocatalytic performance compared to individual TiO2 and InVO4 nanoparticles, achieving a degradation efficiency of 97.18 % within 120 min. Kinetic studies revealed that the photocatalytic degradation of rifampicin by TiO2/InVO4 followed a pseudo-first-order reaction, as indicated by a high R2 value and a reaction rate constant (k) of 9.34 × 10−3. A radical scavenger test identified the primary reactive species involved in the degradation process, showing that holes (h+) were the most crucial, followed by hydroxyl (•OH) and superoxide (•O2−) radicals. The material also demonstrated stability and reusability, maintaining 84.65 % of its initial activity after fifth successive cycles. The escalation of photocatalytic activity could be attributable to a reduced band gap compared to pure TiO2, enabling visible light absorption. This study addresses the pressing issue of pharmaceutical pollution by developing a highly efficient photocatalyst for the degradation of rifampicin, a persistent organic pollutant that poses severe threats to aquatic ecosystems and human health.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemosphere
环境科学-环境科学
CiteScore
15.80
自引率
8.00%
发文量
4975
审稿时长
3.4 months
期刊介绍:
Chemosphere, being an international multidisciplinary journal, is dedicated to publishing original communications and review articles on chemicals in the environment. The scope covers a wide range of topics, including the identification, quantification, behavior, fate, toxicology, treatment, and remediation of chemicals in the bio-, hydro-, litho-, and atmosphere, ensuring the broad dissemination of research in this field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


