妊娠期细颗粒物暴露与自发性早产:利用胎盘转录组和代谢组特征阐明机制。
IF 2.5
2区 医学
Q2 DEVELOPMENTAL BIOLOGY
引用次数: 0
摘要
背景:本研究旨在评估胎盘转录组学和代谢组学变化在妊娠期暴露于细颗粒物和自发性早产(sPTB)之间的中介作用。方法:本研究包括来自细胞损伤和早产研究的72名参与者。使用与产妇分娩地址相关的融合1公里分辨率模型输出,估算了怀孕前20周PM2.5和黑碳的每日暴露量。高暴露定义为PM2.5 9 μg/m3或黑碳1 μg/m3的天数,按月计算。结果:妊娠3-5个月的黑碳暴露与sPTB的发病率增加有关。我们确定了63个与黑碳相关的基因和50个与PM2.5相关的基因,主要与细胞应激、免疫反应和发育途径有关。对于黑碳,23个基因在多个暴露窗口中表现出一致的相关性(9个基因始终呈负相关,13个基因始终呈正相关)。MPO、ELANE和GNRH2基因表达的增加介导了黑碳暴露与sPTB增加之间的关联。结论:妊娠期暴露于黑碳与sPTB发病率增加有关,这种关联是通过改变胎盘基因表达介导的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
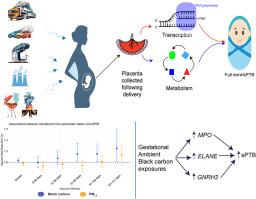
Gestational fine particulate matter exposures and spontaneous preterm birth: Elucidating mechanisms using placental transcriptome and metabolome signatures
Background
This study aims to evaluate the mediating role of placental transcriptomic and metabolomic changes in the association between gestational exposure to fine particulate matter and spontaneous preterm birth (sPTB).
Methods
This study includes 72 participants from the CellulaR Injury and Preterm Birth Study. Daily exposure to PM2.5 and black carbon during the first 20 weeks of pregnancy was estimated using fused 1-km resolution model outputs linked to maternal delivery addresses. High exposure was defined as days with PM2.5 >9 μg/m3 or black carbon >1 μg/m3, calculated monthly across gestation. Early sPTB (<32 weeks) and covariates were extracted from electronic health records. Associations between high-exposure days and expression of 432 sPTB-related genes were evaluated using gene-wise negative binomial models, and associations with 866 placental metabolites were assessed using multiple linear regression. Both analyses adjusted for covariates, using q < 0.2 for statistical significance. High-dimensional mediation analysis was conducted on genes/metabolites with at least one significant hit (q < 0.05).
Results
Black carbon exposure during gestational months 3–5 was associated with increased odds of sPTB. We identified 63 genes linked to black carbon and 50 to PM2.5, mainly related to cellular stress, immune response, and developmental pathways. For black carbon, 23 genes showed consistent associations across multiple exposure windows (9 genes consistently negatively associated, 13 positively associated). An increase in expression of genes MPO, ELANE, and GNRH2 mediated the association between black carbon exposure and increased sPTB.
Conclusion
Gestational exposure to black carbon is associated with increased odds of sPTB, and this association was mediated by altered placental gene expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Placenta
医学-发育生物学
CiteScore
6.30
自引率
10.50%
发文量
391
审稿时长
78 days
期刊介绍:
Placenta publishes high-quality original articles and invited topical reviews on all aspects of human and animal placentation, and the interactions between the mother, the placenta and fetal development. Topics covered include evolution, development, genetics and epigenetics, stem cells, metabolism, transport, immunology, pathology, pharmacology, cell and molecular biology, and developmental programming. The Editors welcome studies on implantation and the endometrium, comparative placentation, the uterine and umbilical circulations, the relationship between fetal and placental development, clinical aspects of altered placental development or function, the placental membranes, the influence of paternal factors on placental development or function, and the assessment of biomarkers of placental disorders.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


