多糖在炎症中的治疗潜力:目前的见解和未来的方向。
IF 4.7
2区 医学
Q2 IMMUNOLOGY
引用次数: 0
摘要
来自植物、微生物、藻类和动物的多糖具有显著的抗炎潜力,为炎症性肠病和关节炎等疾病提供了安全有效的治疗选择。本文综述了它们的来源、结构修饰和机制,包括NF-κB和MAPK通路的调节。在体外模型(如RAW264.7巨噬细胞、THP-1细胞)中,在50-200 μg/mL剂量下,促炎细胞因子(如TNF-α和IL-1β)减少30- 40%,而在体内模型(如dss诱导的结肠炎、胶原诱导的关节炎)中,在50-200 mg/kg剂量下,疾病活动指数降低,组织病理学结果改善。化学修饰(如磺化)可增强生物活性,但生物利用度、方法不一致和剂量变化等挑战仍然存在。综合表3总结了来源、化合物、模型、剂量和影响,而关键的评估强调了方法上的局限性,并提出了标准化的分析方法(例如,ELISA、qPCR)。未来的方向包括纳米技术用于改善递送,生物信息学用于结构优化,以及随机临床试验来验证临床前发现,为新的基于多糖的治疗铺平道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
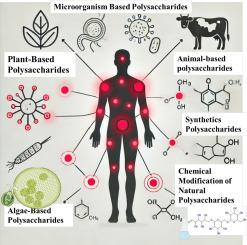
Therapeutic potential of polysaccharides in inflammation: current insights and future directions
Polysaccharides from plants, microorganisms, algae, and animals exhibit significant anti-inflammatory potential, offering safe and effective therapeutic options for diseases like inflammatory bowel disease and arthritis. This review synthesizes their sources, structural modifications, and mechanisms, including modulation of NF-κB and MAPK pathways. In vitro models (e.g., RAW264.7 macrophages, THP-1 cells) demonstrate reductions in pro-inflammatory cytokines (e.g., TNF-α and IL-1β by 30–40 % at doses of 50–200 μg/mL), while in vivo models (e.g., DSS-induced colitis, collagen-induced arthritis) show decreased Disease Activity Index and improved histopathological outcomes at doses of 50–200 mg/kg. Chemical modifications, such as sulfation, enhance bioactivity, though challenges like bioavailability, inconsistent methodologies, and variable dosing persist. A comprehensive Table 3 summarizes sources, compounds, models, doses, and effects, while critical evaluations highlight methodological limitations and propose standardized assays (e.g., ELISA, qPCR). Future directions include nanotechnology for improved delivery, bioinformatics for structural optimization, and randomized clinical trials to validate preclinical findings, paving the way for novel polysaccharide-based therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
8.40
自引率
3.60%
发文量
935
审稿时长
53 days
期刊介绍:
International Immunopharmacology is the primary vehicle for the publication of original research papers pertinent to the overlapping areas of immunology, pharmacology, cytokine biology, immunotherapy, immunopathology and immunotoxicology. Review articles that encompass these subjects are also welcome.
The subject material appropriate for submission includes:
• Clinical studies employing immunotherapy of any type including the use of: bacterial and chemical agents; thymic hormones, interferon, lymphokines, etc., in transplantation and diseases such as cancer, immunodeficiency, chronic infection and allergic, inflammatory or autoimmune disorders.
• Studies on the mechanisms of action of these agents for specific parameters of immune competence as well as the overall clinical state.
• Pre-clinical animal studies and in vitro studies on mechanisms of action with immunopotentiators, immunomodulators, immunoadjuvants and other pharmacological agents active on cells participating in immune or allergic responses.
• Pharmacological compounds, microbial products and toxicological agents that affect the lymphoid system, and their mechanisms of action.
• Agents that activate genes or modify transcription and translation within the immune response.
• Substances activated, generated, or released through immunologic or related pathways that are pharmacologically active.
• Production, function and regulation of cytokines and their receptors.
• Classical pharmacological studies on the effects of chemokines and bioactive factors released during immunological reactions.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


