花椒中具有细胞毒活性的生物碱。
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
从nitidum花椒中分离出了两个先前未被描述的生物碱,8-O-demethylmaclekarpine D(1)和zananthonitine(2),以及一对不可分离的旋体,4-羟基-beecheyamide (3a)和(E)-3-(4-羟基-3-甲氧基苯基)- n -(4-甲氧基苯基)- n -甲基丙烯酰胺(3b)。这些化合物含有35种已知的生物碱,包括苯并苯胺类(4-21)、喹啉类(22-31)、阿啡类(32-35)和其他结构类型(36-38)。通过全面的光谱分析,包括NMR, hresms, x射线晶体学,ECD和计算的13CNMR DP4+分析,实现了结构解析。细胞毒性评价显示,化合物6、7、18和33 (IC50 = 7.29-22.90 μM)对HepG2细胞的增殖具有较强的抑制作用,优于阿霉素(IC50 = 28.92 ± 0.48 μM)。同样,化合物9、16和21 (IC50 = 21.77 ~ 25.13 μM)对SW480细胞的抑制作用优于阿霉素(IC50 = 31.15 ± 0.24 μM)。本文章由计算机程序翻译,如有差异,请以英文原文为准。
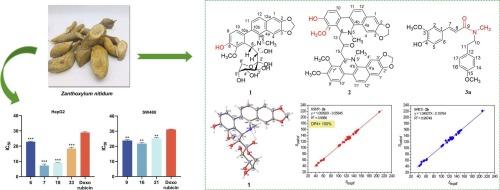
Alkaloids with cytotoxic activities from Zanthoxylum nitidum
Two previously undescribed alkaloids, 8-O-demethylmaclekarpine D (1) and zanthonitine (2), along with a pair of inseparable rotamers, 4-hydroxyl-beecheyamide (3a) and (E)-3-(4-hydroxy-3-methoxyphenyl)-N-(4-methoxyphenethyl)-N-methylacrylamide (3b), were isolated from Zanthoxylum nitidum. These compounds were accompanied by 35 known alkaloids, including benzophenanthridines (4–21), quinolines (22−31), aporphines (32–35) and other structural types (36–38). Structural elucidation was achieved through comprehensive spectroscopic analyses, including NMR, HRESIMS, X-ray crystallography, ECD and calculated 13C NMR DP4+ analysis. Cytotoxicity evaluation revealed compounds 6, 7, 18 and 33 (IC50 = 7.29–22.90 μM) exhibited potent inhibitory effects on the proliferation of HepG2 cells, outperforming the efficacy of doxorubicin (IC50 = 28.92 ± 0.48 μM). Similarly, compounds 9, 16 and 21 (IC50 = 21.77–25.13 μM) suppressed SW480 cells, superior to doxorubicin (IC50 = 31.15 ± 0.24 μM).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


