新型1,3-苯二唑衍生物抗肿瘤活性的设计、合成和评价。
IF 2.6
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
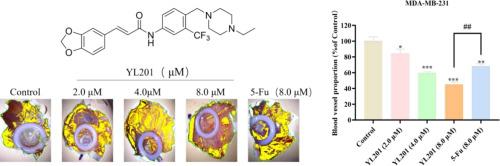
本研究设计了新的1,3-苯二唑衍生物(胡椒碱活性必需的核心部分),通过:1)保留1,3-苯二唑环(抗肿瘤疗效的关键);2)引入乙烯基连接剂(根据我们之前的工作,与直接酰胺连接相比,活性增强);3)加入三氟甲基哌嗪(经证实可增强抗肿瘤作用)。经溴化、亲核取代、还原、缩合等步骤合成目标化合物,其结构经1H NMR、13C NMR和HR-MS证实。经溴化、亲核取代、还原、缩合等步骤合成目标化合物,其结构经1H NMR、13C NMR和HR-MS证实。MTT试验显示,大多数衍生物对HeLa、A498和MDA-MB-231细胞具有显著的细胞毒性。其中,(E) 3 -(苯并[d] [1,3] dioxol-5-yl) - n - (4 - ((4-ethylpiperazin-1-yl)甲基)3 - (trifluoromethyl)苯基)丙烯酰胺(YL201)最强的活动对mda - mb - 231细胞(IC₅₀ = 4.92±1.09 μM),优于5 -氟尿嘧啶(研究者用、IC₅₀ = 18.06±2.33 μM)。体外实验(菌落形成、粘附、Transwell侵袭、伤口愈合)显示YL201浓度依赖性地抑制MDA-MB-231细胞的增殖、粘附、侵袭和迁移(例如,8 μM YL201导致23.30 ± 1.20 %的增殖率,而5-Fu的增殖率为69.63 ± 3.63 %)。在鸡胚毛囊尿囊膜(CAM)异种移植中,YL201抑制肿瘤血管生成,降低肿瘤重量(8 μM时为8.17 ± 1.17 mg, 5-Fu时为14.40 ± 1.05 mg),而不影响鸡胚重量,表明良好的体内安全性。SwissADME预测显示YL201符合关颖杉的规则五(461.48 MW = ,logP = 3.94 HBA = 8,HBD = 1,可旋转的公债 = 8),表明druggability有利。综上所述,YL201是一种有前景的乳腺癌治疗候选药物,值得进一步的临床前研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis and evaluation of novel 1,3-benzodioxole derivatives for their anti-tumor activity
This study designed novel 1,3-benzodioxole derivatives (a core moiety of piperine essential for activity) by: 1) Retaining the 1,3-benzodioxole ring (critical for antitumor efficacy); 2) Introducing a vinyl linker (enhanced activity vs. direct amide attachment, per our prior work); 3) Incorporating trifluoromethylpiperazine (proven to boost antitumor effects). Target compounds were synthesized via bromination, nucleophilic substitution, reduction, and condensation, with structures confirmed by 1H NMR, 13C NMR, and HR-MS.
Target compounds were synthesized via bromination, nucleophilic substitution, reduction, and condensation, with structures confirmed by 1H NMR, 13C NMR, and HR-MS. MTT assay showed most derivatives exhibited significant cytotoxicity against HeLa, A498, and MDA-MB-231 cells. Among them, (E)-3-(benzo[d][1,3]dioxol-5-yl)-N-(4-((4-ethylpiperazin-1-yl)methyl)-3-(trifluoromethyl)phenyl)acrylamide (YL201) had the strongest activity against MDA-MB-231 cells (IC₅₀ = 4.92 ± 1.09 μM), outperforming 5-fluorouracil (5-Fu, IC₅₀ = 18.06 ± 2.33 μM).
In vitro assays (colony formation, adhesion, Transwell invasion, wound healing) demonstrated YL201 concentration-dependently suppressed MDA-MB-231 cell proliferation, adhesion, invasion, and migration (e.g., 8 μM YL201 led to 23.30 ± 1.20 % proliferation rate vs. 69.63 ± 3.63 % for 5-Fu). In chick embryo chorioallantoic membrane (CAM) xenografts, YL201 inhibited tumor angiogenesis and reduced tumor weight (8.17 ± 1.17 mg at 8 μM vs. 14.40 ± 1.05 mg for 5-Fu) without affecting chick embryo weight, indicating good in vivo safety.
SwissADME prediction showed YL201 complies with Lipinski's Rule of Five (MW = 461.48, logP = 3.94, HBA = 8, HBD = 1, rotatable bonds = 8), suggesting favorable druggability. In conclusion, YL201 is a promising candidate for breast cancer treatment worthy of further preclinical research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


