用功能脂质体纳米药物增强脊髓损伤恢复:一种神经保护和抗炎方法。
IF 4.7
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
背景:脊髓损伤(SCI)会引发严重的继发性损伤和持续的神经功能缺陷,例如不可接受的高度功能丧失,其中bbbb80的患者会经历不可逆的下肢瘫痪。方法:为了解决这些挑战,我们开发了RLNPP@Tha,一种靶向cd44的ros反应脂质体纳米药物,选择性地将沙利度胺递送到SCI微环境中。研究了RLNPP@Tha的理化性质、药物释放、细胞摄取和抗炎/神经保护作用。在大鼠脊髓损伤模型中评估其治疗效果,包括运动恢复、炎症调节、组织修复和生物安全性。结果:RLNPP@Tha表现出ros依赖性的沙利度胺释放(在400 μM H₂O₂下,12小时内释放92%),并被激活的BV2小胶质细胞选择性摄取。在体外,它减少了细胞内ROS约70%,抑制了促炎细胞因子(IL-1β, IL-6, TNF-α) 50-70%,并保护SH-SY5Y神经元免于氧化凋亡(85%的存活率,而对照组为30%)。在体内,RLNPP@Tha将Basso-Beattie-Bresnahan (BBB)评分提高至18-19(生理盐水为5-6),细胞因子水平降低45-60%,保存脊髓形态,增强神经元存活,抑制胶质瘢痕形成,促进M2小胶质极化。生物安全评估证实了最小的全身毒性。结论:RLNPP@Tha的双CD44/ ros反应机制能够精确调节脊髓损伤炎症微环境,为继发性损伤标志物(设计约束层次的2-4级)提供足够的改善,以潜在地解决1级临床挑战,例如中度挫伤脊髓患者不可接受的功能丧失。这种纳米药物为解决神经保护方面未满足的需求提供了初步证据,为先进的靶向治疗铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
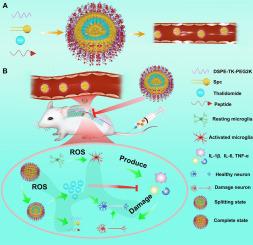
Enhancing spinal cord injury recovery with functional liposomal nanomedicine: A neuroprotective and anti-inflammatory approach
Background
Spinal cord injury (SCI) triggers severe secondary damage and persistent neurological deficits, exemplified by unacceptably high functional loss where >80 % of patients experience irreversible lower limb paralysis with <20 % achieving meaningful recovery, driven by pro-inflammatory M1 microglia that secrete inflammatory cytokines and reactive oxygen species (ROS). Thalidomide’s anti-inflammatory potential is hindered by low bioavailability and systemic toxicity, necessitating targeted delivery strategies to address this unmet Level 1 clinical need for restoring acceptable function in specific SCI scenarios.
Methods
To address these challenges, we developed RLNPP@Tha, a CD44-targeted, ROS-responsive liposomal nanomedicine, to selectively deliver thalidomide to the SCI microenvironment. RLNPP@Tha’s physicochemical properties, drug release, cellular uptake, and anti-inflammatory/neuroprotective effects were characterized in vitro. Its therapeutic efficacy, including locomotor recovery, inflammation modulation, tissue repair, and biosafety, was evaluated in a rat SCI model.
Results
RLNPP@Tha exhibited ROS-dependent thalidomide release (92 % at 400 μM H₂O₂ in 12 h) and selective uptake by activated BV2 microglia. In vitro, it reduced intracellular ROS by ∼70 %, suppressed pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) by 50–70 %, and protected SH-SY5Y neurons from oxidative apoptosis (85 % viability vs. 30 % in controls). In vivo, RLNPP@Tha improved Basso-Beattie-Bresnahan (BBB) scores to 18–19 (vs. 5–6 for saline), reduced cytokine levels by 45–60 %, preserved spinal cord morphology, enhanced neuronal survival, inhibited glial scarring, and promoted M2 microglial polarization. Biosafety assessments confirmed minimal systemic toxicity.
Conclusion
RLNPP@Tha’s dual CD44/ROS-responsive mechanism enables precise modulation of the SCI inflammatory microenvironment, providing sufficient improvements in secondary injury markers (Levels 2–4 of the design constraint hierarchy) to potentially address Level 1 clinical challenges, such as unacceptable functional loss in moderate contusion SCI cases. This nanomedicine offers preliminary evidence toward solving unmet needs in neuroprotection, paving the way for advanced targeted therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


