ROCK2通过作为rna结合蛋白调控PAI-1/NLRP3轴和衰老逃逸来促进肾细胞癌的进展。
IF 3.7
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
摘要
肾细胞癌(RCC)是一种侵袭性恶性肿瘤,晚期治疗选择有限。尽管RCC的发病率在全球范围内不断增加,但其发展的分子机制仍然知之甚少。在这项研究中,我们证明了ROCK2通过转录后调控PAI-1促进RCC的进展。RNA测序和细胞因子分析显示,rock2敲低的RCC细胞PAI-1表达显著降低。PAI-1的过表达恢复NLRP3炎性体的激活,抑制细胞衰老,增强线粒体分裂和自噬,这些作用被NLRP3抑制剂MCC950所消除。在体内,PAI-1过表达促进肿瘤生长,抑制细胞凋亡和衰老,而NLRP3抑制逆转了这些作用。RNA免疫沉淀实验证实ROCK2直接结合PAI-1 mRNA,提示其存在转录后调控机制。总的来说,这些结果支持了ROCK2通过稳定PAI-1 mRNA促进RCC进展的模型,从而激活NLRP3炎症小体,抑制细胞衰老,促进线粒体重塑,这表明ROCK2/PAI-1/NLRP3轴可能作为RCC的潜在治疗靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
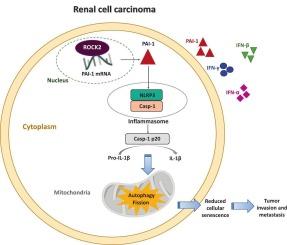
ROCK2 promotes renal cell carcinoma progression by functioning as an RNA-binding protein regulating the PAI-1/NLRP3 axis and senescence escape
Renal cell carcinoma (RCC) is an aggressive malignancy with limited treatment options at advanced stages. Although the incidence of RCC is increasing globally, the molecular mechanisms driving its progression remain poorly understood. In this study, we demonstrate that ROCK2 promotes RCC progression through post-transcriptional regulation of PAI-1. RNA sequencing and cytokine profiling of ROCK2-knockdown RCC cells revealed a significant decrease in PAI-1 expression. Overexpression of PAI-1 restored NLRP3 inflammasome activation, inhibited cellular senescence, and enhanced mitochondrial fission and autophagy, effects that were abolished by the NLRP3 inhibitor MCC950. In vivo, PAI-1 overexpression promoted tumor growth and suppressed both apoptosis and senescence, while NLRP3 inhibition reversed these effects. RNA immunoprecipitation assays confirmed that ROCK2 directly binds to PAI-1 mRNA, suggesting a post-transcriptional regulatory mechanism. Collectively, these results support a model in which ROCK2 facilitates RCC progression by stabilizing PAI-1 mRNA, thereby activating the NLRP3 inflammasome, inhibiting cellular senescence, and promoting mitochondrial remodeling, suggesting that the ROCK2/PAI-1/NLRP3 axis may serve as a potential therapeutic target for RCC.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


