氨基喹啉定向电化学钴催化C(sp²)-H烷氧基化。
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
电化学上,在温和、无氧化的条件下,以8-氨基喹啉为导向基团,用钴(II)催化了二茂铁和芳烃酰胺的C(sp2)-H烷氧基化反应。该方案提供了中高产量的广泛的烷氧基化产品,采用现成的醇作为烷氧基源和绿色溶剂。值得注意的是,该方法通过持续电解实现了高效的C-O键形成,并为烷基化有机化合物提供了一条实用的途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
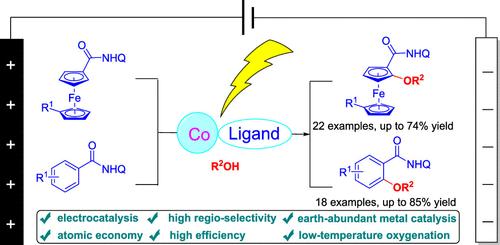
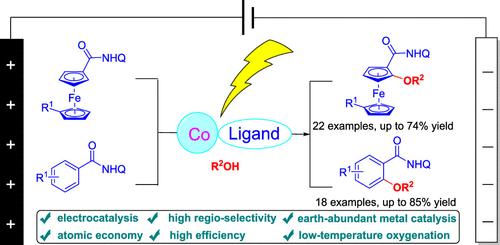
Aminoquinoline-Directed Electrochemical Cobalt-Catalyzed C(sp²)–H Alkoxylation
Electrochemically, earth-abundant cobalt(II)-catalyzed C(sp2)–H alkoxylation of ferrocenyl and arenyl amides with 8-aminoquinoline directing groups was developed under mild, oxidant-free conditions. This protocol afforded a broad range of alkoxylated products in moderate to high yields, employing readily available alcohols as both alkoxy sources and green solvents. Notably, this method enabled efficient C–O bond formation via sustainable electrolysis and offered a practical route to alkylated organic compounds.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


