磁性可分离Co@Co -Al-O催化剂的NaBH4水解:水活化对活性和稳定性的影响
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
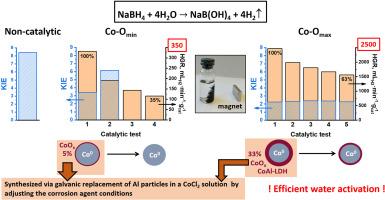
D2O取代H2O过程中的动力学同位素效应(kH/kD, KIE)是评价NaBH4水解过程中水活化的一个有价值的工具。然而,其与催化剂活性和稳定性的关系尚不清楚。本研究通过电替换法合成了两种不同活性的具有Co0核和“Co-Al-O″”壳层的磁可分离催化剂。与非催化过程相比,水在催化剂表面的活化更有效,从而降低了kH/kD。活性Co- omax催化剂含有较多的氧化相(Co3O4、CoO、Co(OH)2、Co- al层状双氧根),这些氧化相在反应介质中还原为活性组分。它们在Co-Omin中的缺乏导致它们在重复使用后活性急剧下降,kH/kD (>5)急剧增加,表明它们向非催化途径转变。相比之下,高活性的Co-Omax表现出高效的催化水活化,在5个循环中稳定的kH/kD为2.34±0.05。观察到该催化剂的氢生成速率单调下降可能归因于氢化物活化步骤。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Magnetically separable Co@Co–Al–O catalysts for NaBH4 hydrolysis: Water activation impact on activity and stability
The kinetic isotope effect (kH/kD, KIE) during replacement of H2O with D2O is a valuable tool for evaluating water activation in NaBH4 hydrolysis. However, its correlation with catalyst activity and stability remains unclear. In this study, two magnetically separable catalysts of different activity with a Co0 core and a “Co–Al–O″ shell were synthesized via galvanic replacement. Compared to the non-catalytic process, water activation on the catalyst surface occurs more efficiently, resulting in reduced kH/kD. The active Co-Omax catalyst contains a higher amount of oxidized phases (Co3O4, CoO, Co(OH)2, Co–Al layered double hydroxide), which reduce to the active component in the reaction medium. Their deficiency in Co-Omin causes a sharp activity decline and a drastic increase in kH/kD (>5) upon reuse, indicating a shift toward the non-catalytic pathway. In contrast, highly active Co-Omax demonstrates an efficient catalytic water activation supported by a stable kH/kD of 2.34 ± 0.05 over five cycles. The observed monotonic decrease in H2 generation rate for this catalyst is probably attributable to the hydride activation step.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


