新型MBP/Fe2+/PMS工艺协同降解水中头孢克肟
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
摘要
头孢克肟(CFX)是一种应用广泛的抗生素,已成为水体抗生素污染治理的挑战。本研究比较了微鼓泡等离子体(MBP)联合铁离子(Fe2+)激活过氧单硫酸根(PMS)和过氧二硫酸根(PDS)降解CFX的性能和机理。研究了PMS/PDS和Fe2+用量、等离子体放电电压和气体流量对降解效率的影响。在最优条件下,CFX的降解率达到90.75 %,显著高于MBP单独(27.81 %)和MBP/PDS体系(23.76 %),表明MBP、Fe2+和PMS之间存在高度协同作用。该系统的能源效率高达1.95 g/kWh。紫外可见光谱和总有机碳分析表明,PMS和Fe2+的加入促进了CFX的降解和矿化。活性氧和氮的检测结合自由基清除实验证实,单线态氧(1O2)、羟基自由基(·OH)和硫酸盐自由基(SO4·−)是降解过程中最重要的活性物质。根据液相色谱-质谱分析结果,确定了CFX的三种主要降解途径。此外,利用毒性评价软件对CFX及其降解中间体进行毒性评价。本研究提供了一种基于等离子体降解CFX的新方法,对未来抗生素污染水体的处理具有参考价值。本文章由计算机程序翻译,如有差异,请以英文原文为准。
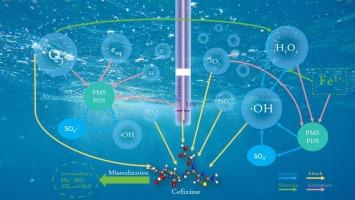
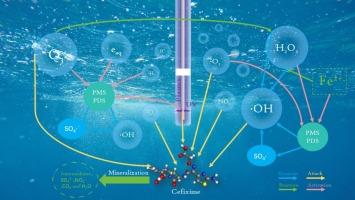
Novel MBP/Fe2+/PMS process for efficient synergistic degradation of cefixime in water
Cefixime (CFX), a widely used antibiotic, has become a challenge for the treatment of antibiotic pollution in water bodies. In this study, the performance and mechanism of microbubbling plasma (MBP) combined with ferrous ions (Fe2+) to activate peroxymonosulfate (PMS) and peroxodisulfate (PDS) in degrading CFX were compared. The effects of the dosages of PMS/PDS and Fe2+, plasma discharge voltage, and gas flow rate on the degradation efficiency were investigated systematically. Under optimal conditions, the degradation rate of CFX reached 90.75 %, which was significantly higher than that of MBP alone (a 27.81 % increase) and the MBP/PDS system (a 23.76 % increase), indicating a high degree of synergy among MBP, Fe2+, and PMS. The energy efficiency of this system was as high as 1.95 g/kWh. Ultraviolet–visible spectroscopy and total organic carbon analysis indicated that the addition of PMS and Fe2+ promoted the degradation and mineralization of CFX. The detection of reactive oxygen and nitrogen species combined with radical scavenging experiments confirmed that singlet oxygen (1O2), hydroxyl radicals (·OH) and sulfate radicals (SO4·−) were the most important active species in the degradation process. Based on the results of a liquid-chromatography–mass-spectrometry analysis, three main CFX degradation pathways were identified. In addition, the toxicities of CFX and its degradation intermediates were evaluated using toxicity assessment software. This study provides a new plasma-based method for the degradation of CFX, which has value as a reference for the future treatment of antibiotic-polluted water bodies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


