真菌脂肪酸合成的变构调节
IF 4.3
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
菌群脂肪酸合成酶(FASs)催化缩合、脱水和还原的迭代循环,以产生饱和脂肪酸。尽管这些多酶是有吸引力的抗真菌药物靶点,但没有临床批准的小分子抑制剂存在,并且对脂肪酸合成的调控仍然知之甚少。在这里,我们发现了棕榈酰辅酶a对FAS酮酰还原反应的变构调节。棕榈酸酯部分结合了酿酒酵母菌和白色念珠菌真菌FAS中央轮的远端位点。该位点也可容纳较短的酰基链,但只有棕榈酰辅酶a抑制酮酰还原酶(KR)活性。虽然还原酶结构域没有发生重大构象变化,但棕榈酰辅酶a结合抑制了中央盘的动力学,提高了局部分辨率并稳定了结构水分子。这种熵效应是还原酶位点变构通信的基础。我们的发现揭示了一种可用于抗真菌药物设计的真菌FAS调控机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
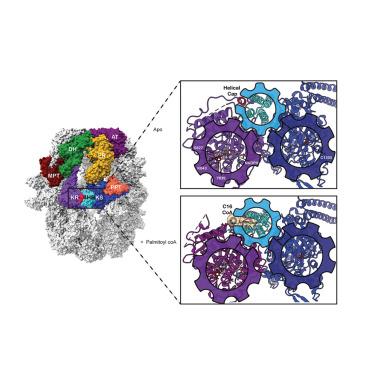
Allosteric regulation of fungal fatty acid synthesis
Mycobiota fatty acid synthases (FASs) catalyze iterative cycles of condensation, dehydration, and reduction to produce saturated fatty acids. Although these multienzymes are attractive antifungal drug targets, no clinically approved small-molecule inhibitors exist, and the regulation of de novo fatty acid synthesis remains poorly understood. Here, we identify an allosteric regulation of the FAS ketoacyl reduction reaction by palmitoyl-CoA. The palmitate moiety binds a distal site on the central wheel of fungal FAS from Saccharomyces cerevisiae and Candida albicans. This site also accommodates shorter acyl chains, but only palmitoyl-CoA suppresses ketoacyl reductase (KR) activity. While no major conformational changes occur in the reductase domain, palmitoyl-CoA binding quenches dynamics in the central disk, improving local resolution and stabilizing structured water molecules. This entropic effect underlies allosteric communication to the reductase site. Our findings uncover a regulatory mechanism of fungal FAS exploitable for antifungal drug design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


